Objective: This study aims to compare the decision made by patients and physicians in selecting device-aided therapy (DAT) for advanced Parkinson’s disease (aPD) and to determine if discordance in patient choices affects treatment outcomes.
Background: Deep Brain Stimulation (DBS), Continuous Subcutaneous Apomorphine Infusion (CSAI) and Levodopa/Carbidopa Intestinal Gel (LCIG) are three available DAT in Thailand for managing aPD. Various factors related to the healthcare system, clinician, patient, and prognosis influence the selection of DAT. Despite numerous studies and guidelines, their applicability to individual patient cases may be questioned. Patient preference is crucial for treatment adherence, given the need for full patient cooperation in initiating and managing DAT.
Method: This retrospective cohort study examined aPD patients who underwent DAT from 2006 to January 2024. Data on demographics, patient and physician decisions, and outcome measures were collected. Subjects were categorized based on concordance and discordance between patient and physician decisions. Differences between patient and physician decisions were analyzed. Subsequently, DAT choices and outcomes were compared between the groups.
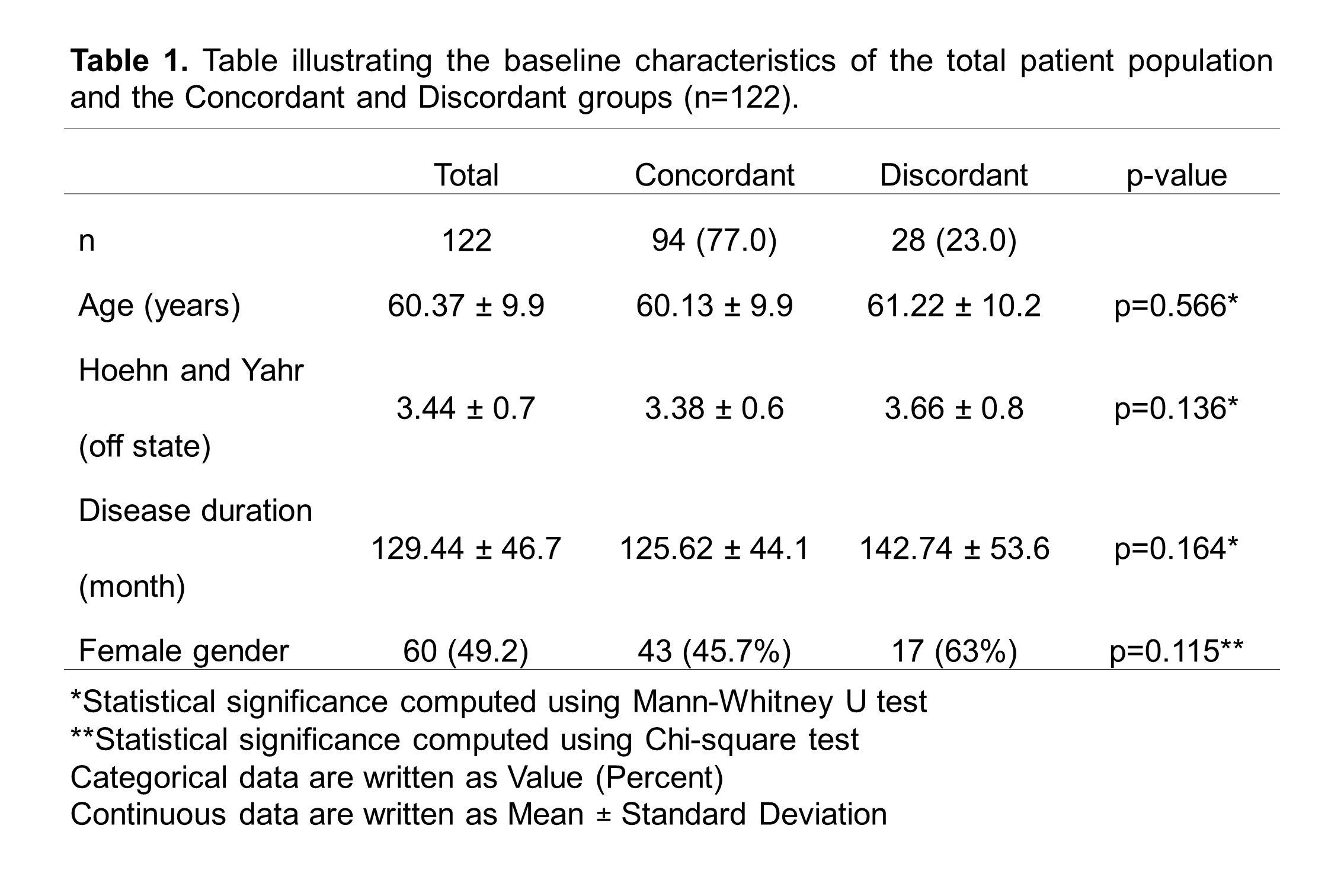
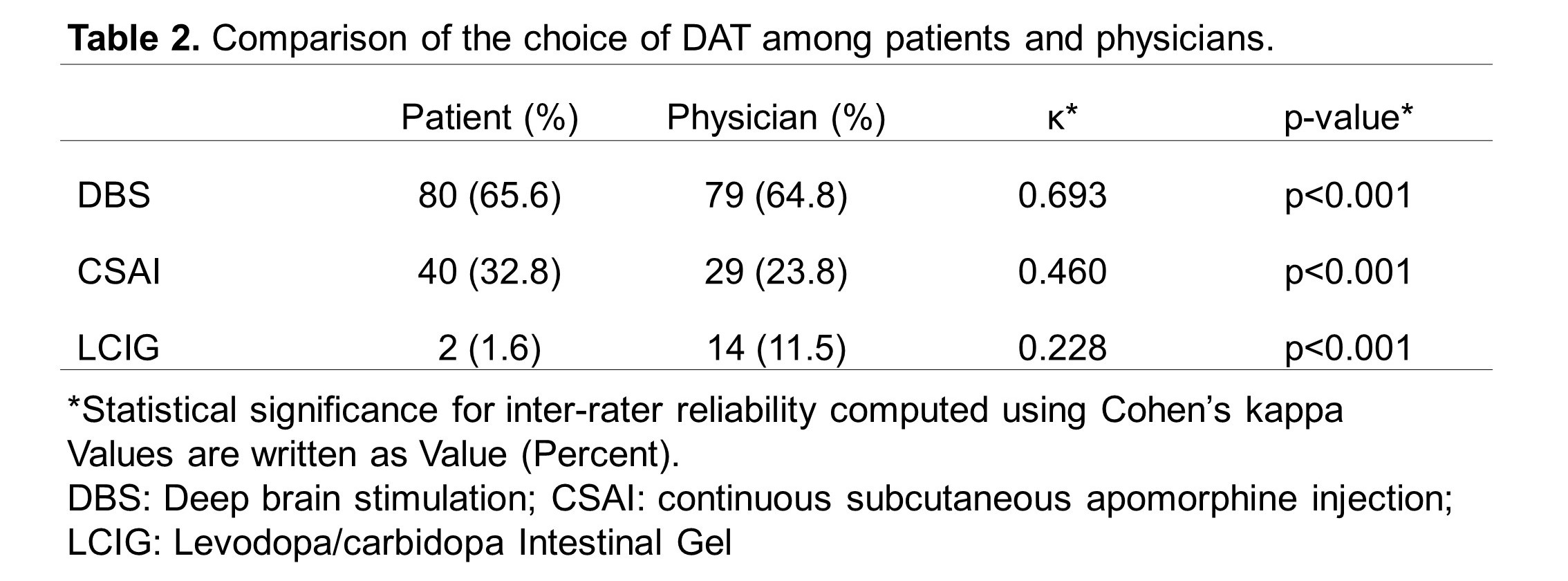
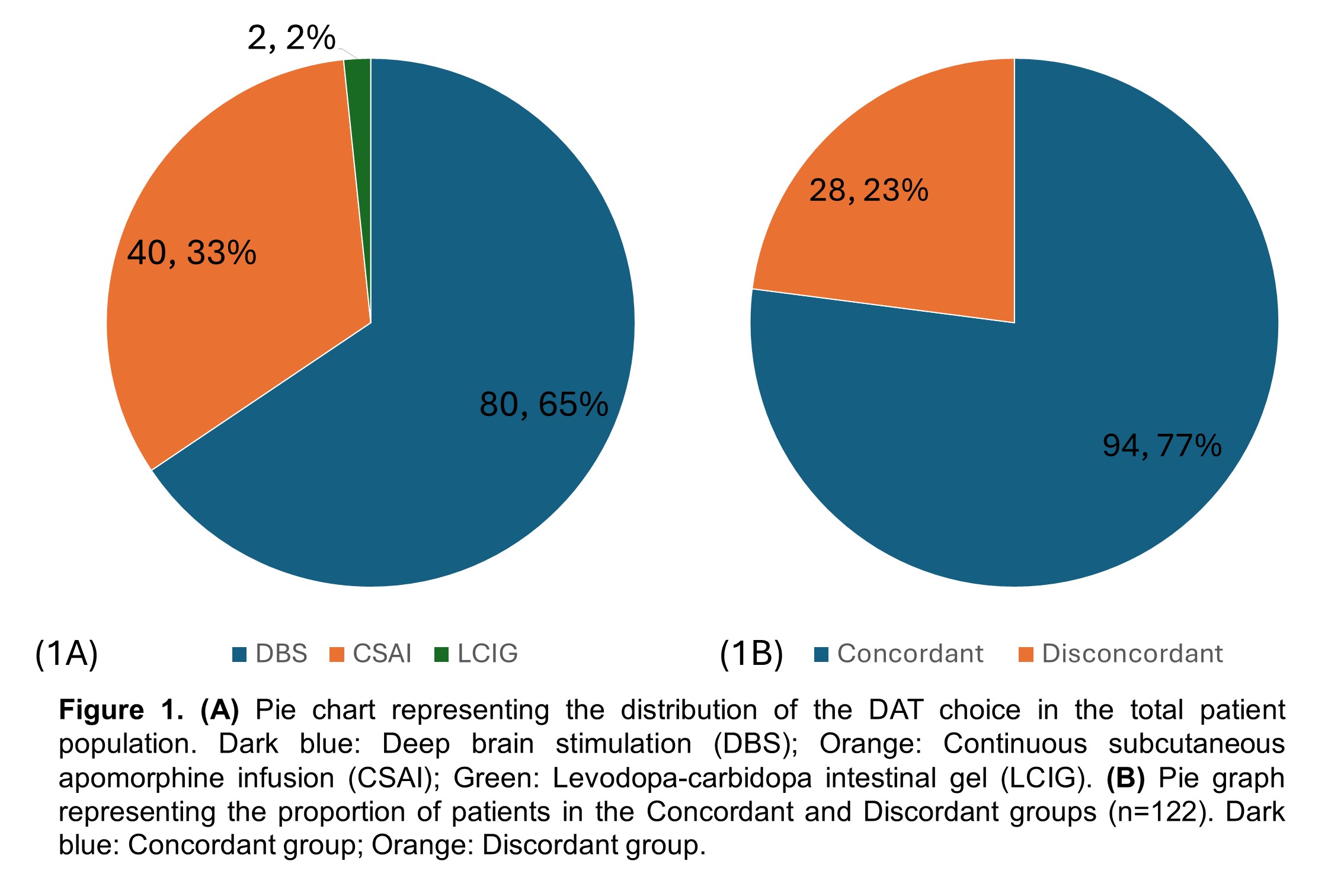
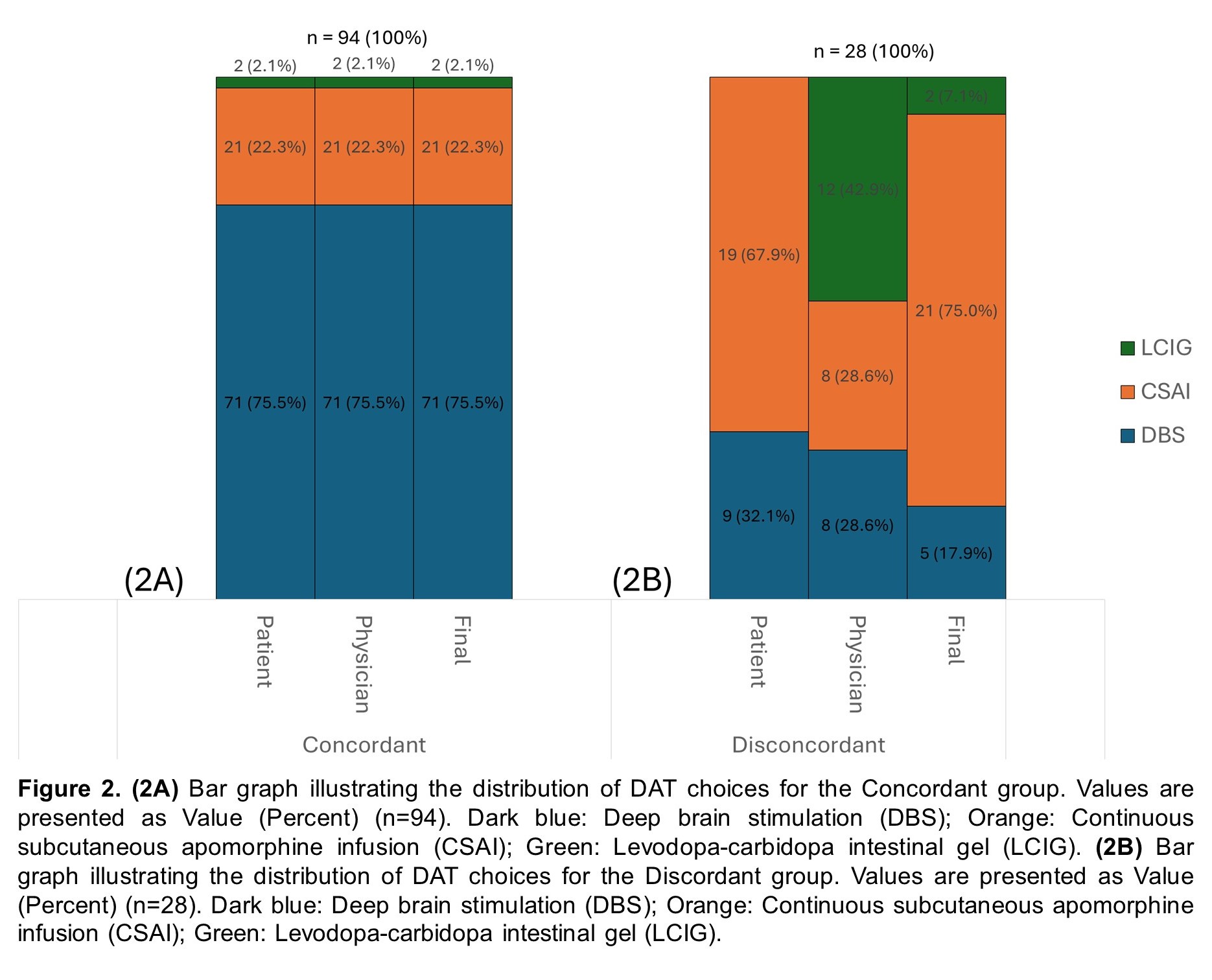
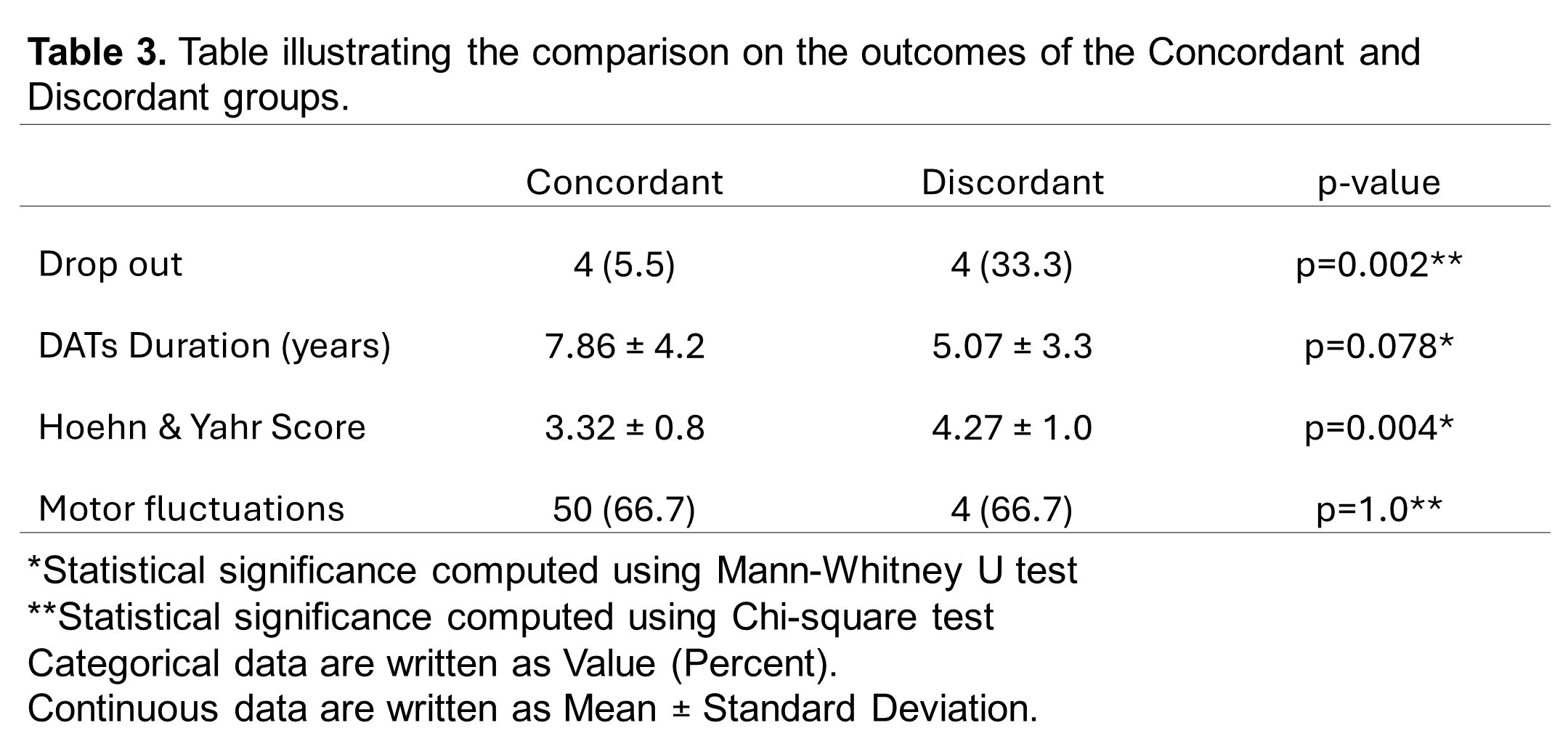
Results: Of the 122 patients who underwent DAT, 94 (77.0%) were in the concordant group, and 28 (23.0%) were in the discordant group [Table 1; Figure 1B]. Baseline characteristics were statistically similar between groups [Table 1]. Overall, both patients (65.6%) and physicians (64.8%) preferred DBS which was reflected in the final implemented modality (62.3%) [Table 2; Figure 1A], consistent with the concordant group [Figure 2A]. However, the discordant group showed increased use of CSAI (75.0%) due to concerns about age, side effects, neuropsychiatric, and balance symptoms [Figure 2B]. Inter-rater reliability analysis revealed moderate agreement between patient and physician decision but the highest is with DBS (κ=0.693) and least with LCIG (κ=0.228) [Table 2]. Comparison of outcomes showed that the discordant group has a significantly higher dropout rate (p=0.002) and greater motor impairment represented by Hoehn & Yahr Score (p=0.004) [Table 3].
Conclusion: There is discordance between patient and physician choices for DAT, but DBS remained the most preferred and implemented options. Such discordance in patient choice correlates with increased drop-out rates and motor impairment.
Table 1
Table 2
Figure 1
Figure 2
Table 3
References: [1] Marsili L, Bologna M, Miyasaki JM, Colosimo C. Device-aided therapies for advanced Parkinson disease: insights from an international survey. Neurol Sci. 2021;42(7):2961-4., [2] Serva SN, Bernstein J, Thompson JA, Kern DS, Ojemann SG. An update on advanced therapies for Parkinson’s disease: From gene therapy to neuromodulation. Frontiers in Surgery. 2022;9., [3] Bhidayasiri R, Phokaewvarangkul O, Boonpang K, Boonmongkol T, Thongchuem Y, Kantachadvanich N, Garcia Ruiz PJ. Long-term Apomorphine Infusion Users Versus Short-term Users: An International Dual-center Analysis of the Reasons for Discontinuing Therapy. Clin Neuropharmacol. 2019;42(5):172-8., [4] Auffret M, Weiss D, Stocchi F, Verin M, Jost WH. Access to device-aided therapies in advanced Parkinson’s disease: navigating clinician biases, patient preference, and prognostic uncertainty. J Neural Transm (Vienna). 2023;130(11):1411-32., [5] Hilker R, Antonini A, Odin P. What is the best treatment for fluctuating Parkinson’s disease: continuous drug delivery or deep brain stimulation of the subthalamic nucleus? J Neural Transm (Vienna). 2011;118(6):907-14., [6] Trenkwalder C, Chaudhuri KR, Garcia Ruiz PJ, LeWitt P, Katzenschlager R, Sixel-Doring F, et al. Expert Consensus Group report on the use of apomorphine in the treatment of Parkinson’s disease–Clinical practice recommendations. Parkinsonism Relat Disord. 2015;21(9):1023-30., [7] Fabbri M, Rosa MM, Ferreira JJ. Adjunctive Therapies in Parkinson’s Disease: How to Choose the Best Treatment Strategy Approach. Drugs Aging. 2018;35(12):1041-54., [8] Fox SH, Katzenschlager R, Lim SY, Barton B, de Bie RMA, Seppi K, et al. International Parkinson and movement disorder society evidence-based medicine review: Update on treatments for the motor symptoms of Parkinson’s disease. Mov Disord. 2018;33(8):1248-66., [9] Dijk JM, Espay AJ, Katzenschlager R, de Bie RMA. The Choice Between Advanced Therapies for Parkinson’s Disease Patients: Why, What, and When? J Parkinsons Dis. 2020;10(s1):S65-S73., [10] Deuschl G, Antonini A, Costa J, Śmiłowska K, Berg D, Corvol JC, et al. European Academy of Neurology/Movement Disorder Society ‐ European Section guideline on the treatment of Parkinson’s disease: I. Invasive therapies. European Journal of Neurology. 2022;29(9):2580-95., [11] Foltynie T, Bruno V, Fox S, Kuhn AA, Lindop F, Lees AJ. Medical, surgical, and physical treatments for Parkinson’s disease. Lancet. 2024;403(10423):305-24., [12] Treatment Guideline Subcommittee of Taiwan Movement Disorder S. Evidence-Based Taiwan Consensus Recommendations for the treatment of Parkinson’s disease. Acta Neurol Taiwan. 2023;32(3):145-84., [13] Antonini A, Pahwa R, Odin P, Isaacson SH, Merola A, Wang L, et al. Comparative Effectiveness of Device-Aided Therapies on Quality of Life and Off-Time in Advanced Parkinson’s Disease: A Systematic Review and Bayesian Network Meta-analysis. CNS Drugs. 2022;36(12):1269-83.
To cite this abstract in AMA style:
A. de Leon, O. Phokeawvarangkul, R. Bhidayasiri. Comparing the Selected Device-Aided Therapy of Movement Disorder Specialist Team with Patient Selections [abstract]. Mov Disord. 2024; 39 (suppl 1). https://www.mdsabstracts.org/abstract/comparing-the-selected-device-aided-therapy-of-movement-disorder-specialist-team-with-patient-selections/. Accessed July 12, 2025.« Back to 2024 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/comparing-the-selected-device-aided-therapy-of-movement-disorder-specialist-team-with-patient-selections/