Objective: To compare use of antiparkinsonian medications (APM) among early Parkinson disease (PD) patients across clinical study cohorts.
Background: Initiation of APM is a key confounder of measurements of disease progression in PD. Adjustment for levodopa equivalents provides a tool for improving interpretation of changes in motoric symptom severity after APM have been initiated. Comparing the patterns of APM initiation across studies can guide data analysis and improve trial design.
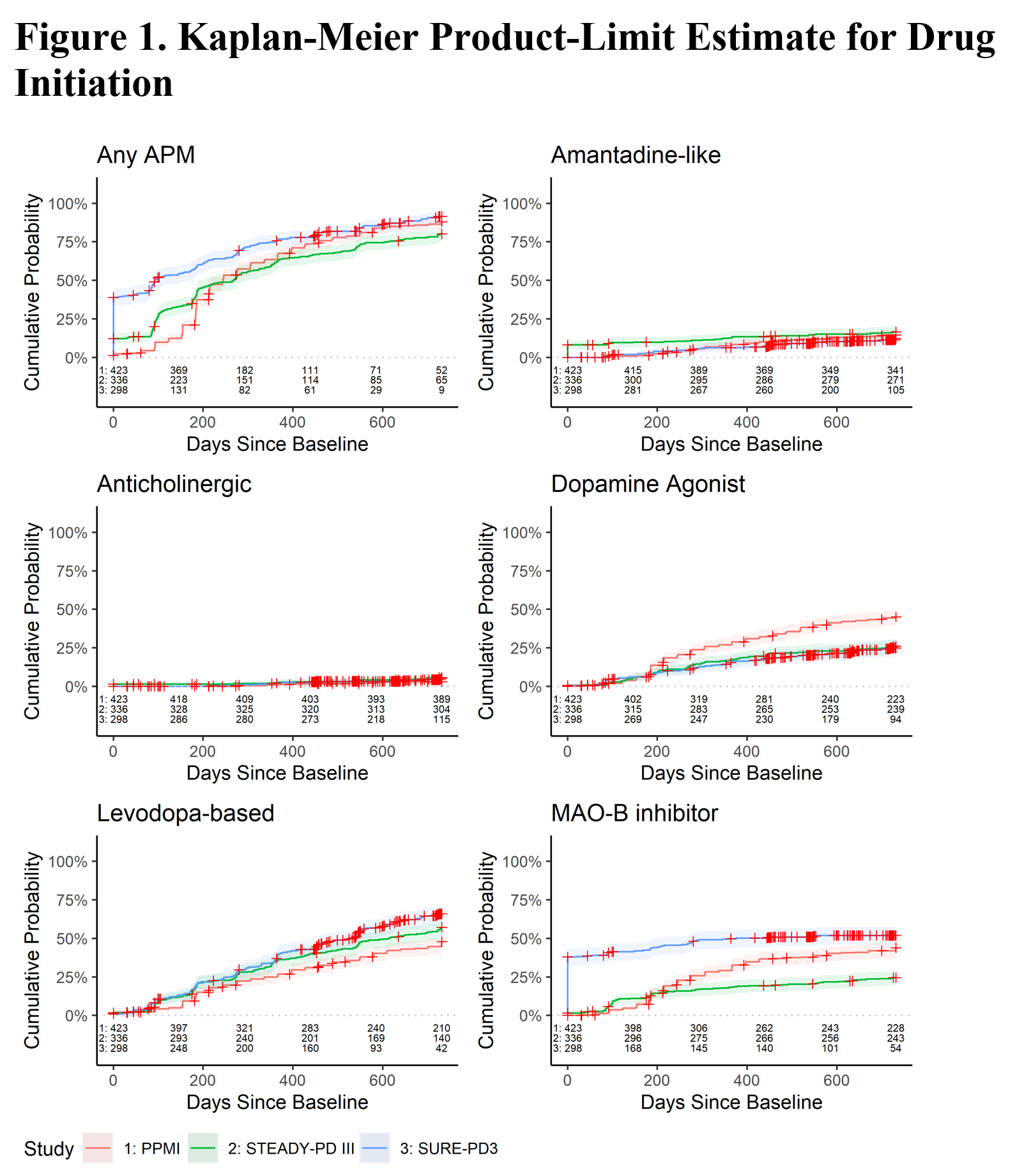
Method: Three independent studies were used for this comparison: 1) de novo PD cohort of the Parkinson’s Progression Markers Initiative (PPMI, NCT01141023), 2) phase 3 randomized placebo controlled trial of isradapine in PD (STEADY-PD III, NCT02168842), 3) phase 3 randomized placebo controlled trial of inosine in PD (SURE-PD3, NCT02642393). Concomitant medication logs were used in each trial to identify and quantify the use of all APM. Daily doses were converted to levodopa equivalent daily dose (LEDD). Studies were compared over the first 2 years after enrollment. Initiation of APM was summarized by Kaplan-Meier estimates by APM category (Amantadine-like, Anticholinergic, Dopamine, Levodopa-based, MAO-B inhibitor) and overall. LEDD was summarized at annual intervals after study initiation.
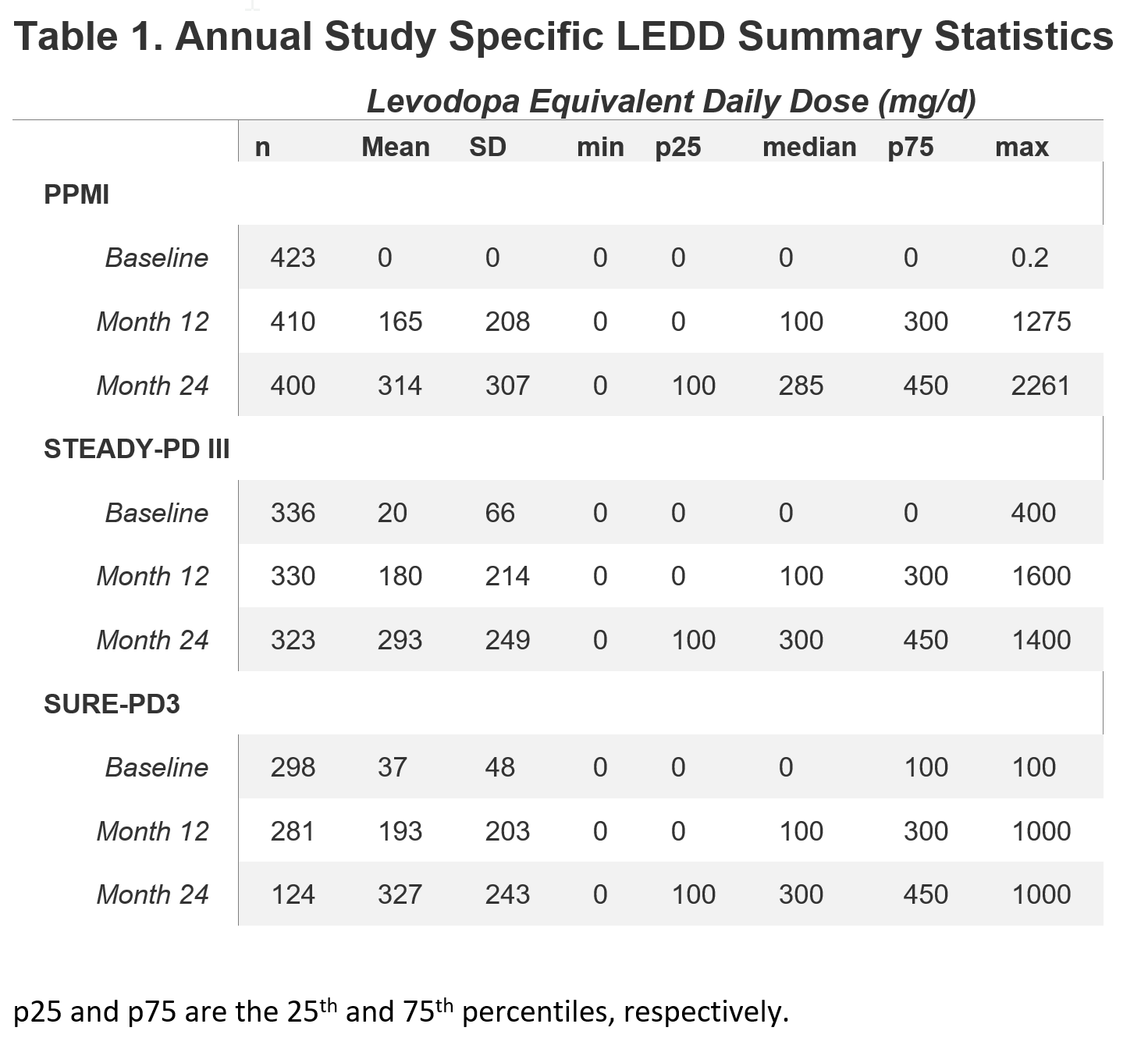
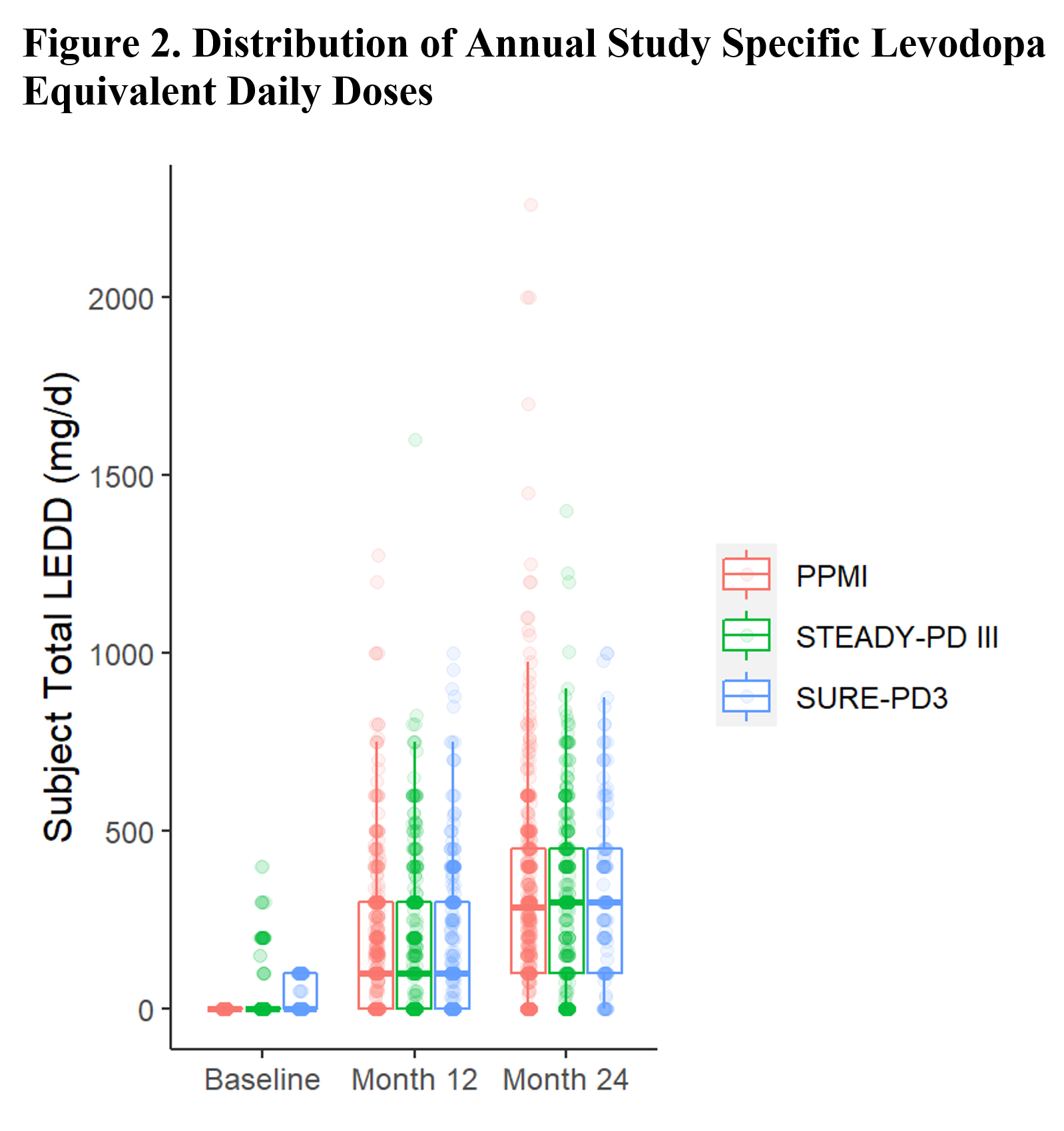
Results: APM use at baseline differed due to differing eligibility criteria: 8% of STEADY-PD III were on amantadine and 1% were on anticholinergics, and 38% of SURE-PD3 were on MAO-B inhibitors. By 2 years of follow-up, the percentage of participants on any APM was similar (PPMI = 84%, STEADY-PD III = 78%, SURE-PD3 = 88%). More PPMI subjects used dopamine agonists while fewer STEADY-PD III subjects used MAO-B inhibitors. While the classes of APM used differed somewhat among studies, LEDD was nearly identical after 1 year (Table 1). Figure 2 shows similar distribution of LEDDs across studies.
Conclusion: Both initiation and standardized dosage of APM are remarkably similar across PD cohorts from an observational study and two clinical trials. This observation suggests that analyses adjusting for LEDD may be generalizable across studies and to the wider PD population.
To cite this abstract in AMA style:
J. Chan, N. Hanan, J. Lowell, M. Kostrzebski, D. Penz, C. Tarolli, T. Mestre, A. Lutz, C. Lungu, C. Coffey, D. Oakes, M. Schwarzschild, T. Simuni, E. Macklin. Comparison of Antiparkinsonian Medication Initiation and Levodopa-Equivalent Daily Dose Across PPMI, STEADY-PD III, and SURE-PD3 [abstract]. Mov Disord. 2021; 36 (suppl 1). https://www.mdsabstracts.org/abstract/comparison-of-antiparkinsonian-medication-initiation-and-levodopa-equivalent-daily-dose-across-ppmi-steady-pd-iii-and-sure-pd3/. Accessed July 2, 2025.« Back to MDS Virtual Congress 2021
MDS Abstracts - https://www.mdsabstracts.org/abstract/comparison-of-antiparkinsonian-medication-initiation-and-levodopa-equivalent-daily-dose-across-ppmi-steady-pd-iii-and-sure-pd3/