Category: Parkinson’s Disease: Clinical Trials
Objective: Evaluate participants’ perceptions of their confidence when engaging in daily activities and interpersonal relationships while using CSAI therapy, relative to the period before CSAI use.
Background: CSAI has been used worldwide to treat motor fluctuations in people with Parkinson disease. The open-label, phase 3, long-term, InfusON trial (NCT02339064) provided safety and efficacy data to support the application for its use in the United States.
Method: This moderator-guided survey was conducted between January and March 2020, and included remaining participants in the ongoing InfusON trial Extension Period. Consenting participants answered questions regarding their current experiences with CSAI therapy (relative to the period before CSAI initiation), the perceived burden of their PD treatment regimen, and their confidence when engaging in physical, social, occupational, and personal care activities, and in their ability to drive, perform work activities, household chores, and function independently without reliance on family or care partners.
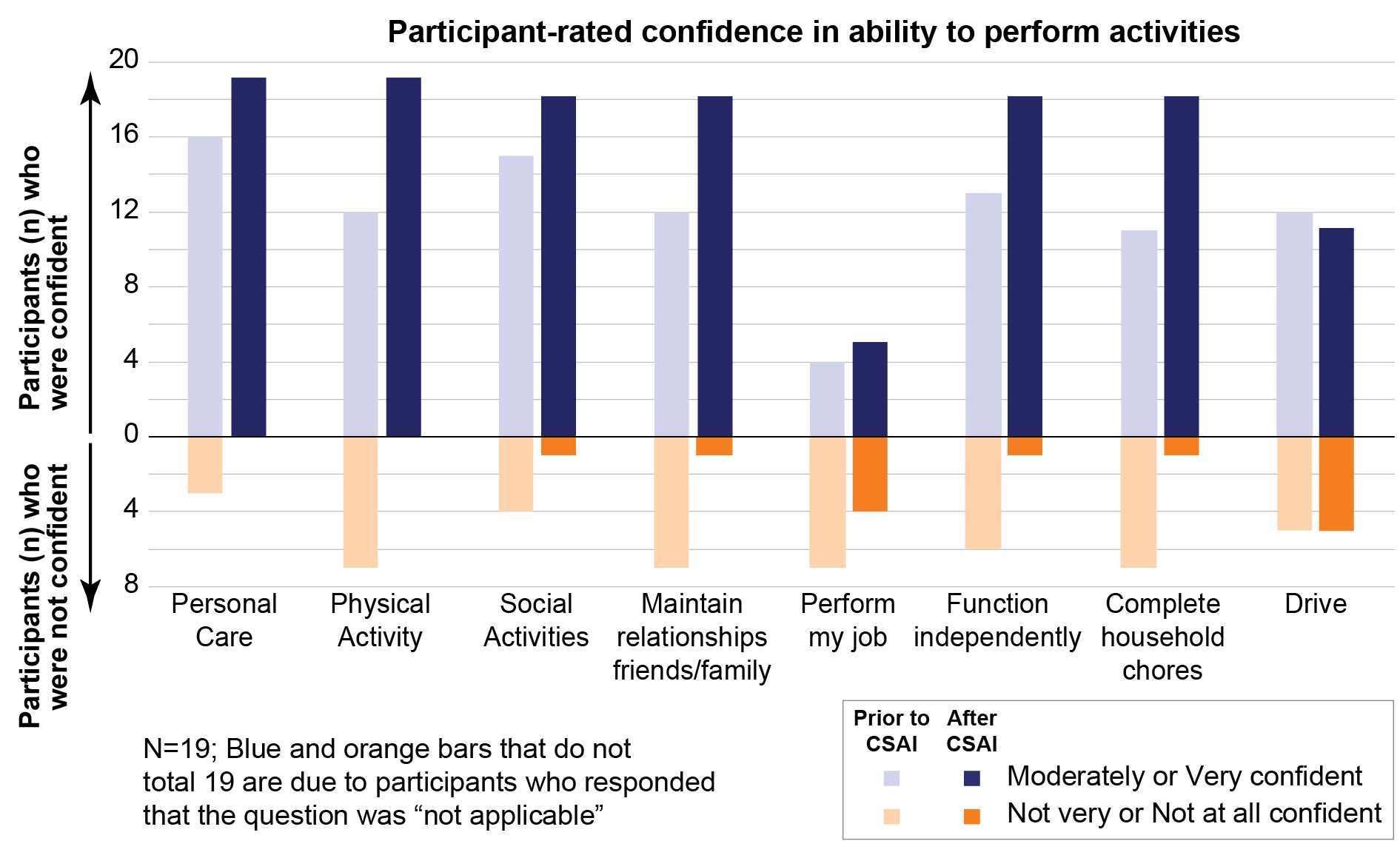
Results: Among the 99 participants enrolled in InfusON, 23 remained in the study at the time of survey conduct, and 19 (82.6%) were available and consented to be interviewed. All survey participants had completed the 52-week study Maintenance Period and were continuing CSAI during the study Extension Period. Despite disease progression and CSAI being used as an add-on therapy, fewer participants (47.4%) rated their PD medication regimen (including CSAI) to be moderately, very, or extremely burdensome compared to their PD medication regimen before CSAI initiation (78.9%). As a whole, participants perceived themselves more confident in their ability to engage in the assessed activities during CSAI use compared with prior to CSAI use. [figure1] Limitations of the analysis include recall bias for experiences prior to CSAI use, and the enriched nature of the sample that did not include individuals who dropped out prior to the time of survey conduct.
Conclusion: Participants using CSAI as adjunctive therapy for OFF episodes in this multi-year extension to a long-term, open-label study perceived lower PD treatment burden, and greater confidence in pursuing social relationships, personal care, and everyday activities, relative to their experiences before CSAI use.
Figure1
To cite this abstract in AMA style:
P. Agarwal, A. Formella, N. Crouse, A. Breiteneicher, M. Grall. Continuous Subcutaneous Apomorphine Infusion (CSAI) Improved Confidence When Engaging in Everyday Activities: Survey of InfusON Study Participants [abstract]. Mov Disord. 2024; 39 (suppl 1). https://www.mdsabstracts.org/abstract/continuous-subcutaneous-apomorphine-infusion-csai-improved-confidence-when-engaging-in-everyday-activities-survey-of-infuson-study-participants/. Accessed June 30, 2025.« Back to 2024 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/continuous-subcutaneous-apomorphine-infusion-csai-improved-confidence-when-engaging-in-everyday-activities-survey-of-infuson-study-participants/