Session Information
Date: Saturday, October 6, 2018
Session Title: Parkinson’s Disease: Clinical Trials, Pharmacology And Treatment
Session Time: 1:45pm-3:15pm
Location: Hall 3FG
Objective: To assess the efficacy and safety of safinamide as an add-on therapy to levodopa in Parkinson patients.
Background: Long-term use of levodopa is associated with dyskinesia and motor symptoms which are generally managed by adding MAO-B or COMT inhibitor. Safinamide is a selective MAO-B inhibitor recently approved by US Food Drug Administration (FDA) as an adjunctive treatment for Parkinson. So, this systematic review of randomized controlled trial (RCT) is aiming to assess the efficacy and safety profile of safinamide.
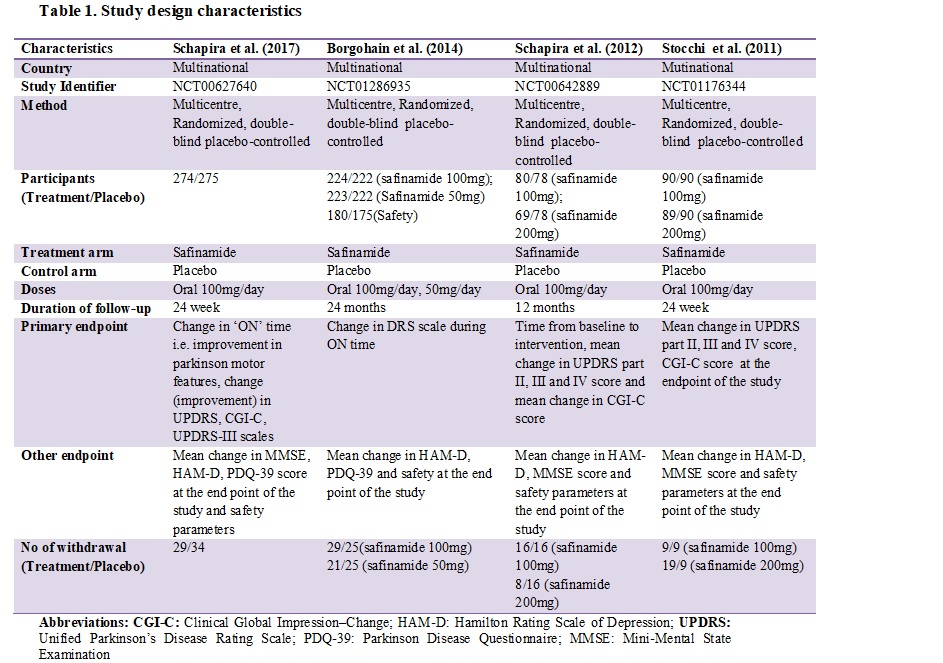
Methods: Electronic databases PubMed and Cochrane central were searched to retrieve the RCTs assessing efficacy and safety of safinamide for inclusion in this systematic review published from the earliest available date to February 2018. Risk of bias among the selected RCTs for inclusion was assessed using Cochrane risk of bias tool. Primary outcomes were mean change in ON time i.e. improvement in parkinson motor features, change in UPDRS, CGI-C, UPDRS-III scales at the endpoint of the study. Secondary outcomes include change in MMSE, HAM-D, PDQ-39 score at the endpoint of the study and safety parameters.
Results: A total of four RCTs qualified the inclusion criteria. Participants were randomized to safinamide 100 mg, 200mg, and placebo. All the included RCTs were multicentre double placebo-controlled in nature. Detailed study design characteristic is presented in table 1. All the included RCTs showed statistically significant improvement in the mean daily ON time without troublesome dyskinesia at the endpoint of the study. There was a statistically significant improvement in CGI-C score, UPDRS part II, III and IV score, HAM-D score in patients randomized to Safinamide 100mg. Dyskinesia was the most common treatment-emergent adverse events in safinamide group reported by two included RCTs.
Conclusions: Safinamide was found to be effective as an adjunct to levodopa in increasing ON time and improvement in motor function in parkinson patients with satisfactory safety profile.
References: 1. Schapira AH, Fox SH, Hauser RA, Jankovic J, Jost WH, Kenney C, Kulisevsky J, Pahwa R, Poewe W, Anand R. Assessment of safety and efficacy of safinamide as a levodopa adjunct in patients with Parkinson disease and motor fluctuations: a randomized clinical trial. JAMA neurology. 2017;74(2):216-24. 2. Borgohain R, Szasz J, Stanzione P, Meshram C, Bhatt MH, Chirilineau D, Stocchi F, Lucini V, Giuliani R, Forrest E, Rice P. Two‐year, randomized, controlled study of safinamide as add‐on to levodopa in mid to late Parkinson’s disease. Movement Disorders. 2014;29(10):1273-80.
To cite this abstract in AMA style:
S. Hussain, A. Najmi. Efficacy and safety of safinamide as a levodopa adjunct in patients with parkinson disease and motor fluctuations: A systematic review of randomized controlled trials [abstract]. Mov Disord. 2018; 33 (suppl 2). https://www.mdsabstracts.org/abstract/efficacy-and-safety-of-safinamide-as-a-levodopa-adjunct-in-patients-with-parkinson-disease-and-motor-fluctuations-a-systematic-review-of-randomized-controlled-trials/. Accessed February 13, 2026.« Back to 2018 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/efficacy-and-safety-of-safinamide-as-a-levodopa-adjunct-in-patients-with-parkinson-disease-and-motor-fluctuations-a-systematic-review-of-randomized-controlled-trials/