Objective: To investigate biological aging in videopolysomnography (vPSG)-confirmed isolated REM Sleep Behavior Disorder (iRBD) patients and vPSG-negative controls, using DNA methylation-based epigenetic clocks.
Background: Isolated RBD is a well-recognized prodromal state of an underlying α-synucleinopathy, occurring several years before a neurodegenerative disorder can be manifest. Epigenetic clocks are mathematical models that, starting from DNA methylation profiles, return an estimate of the age of an individual. The discrepancy between predicted epigenetic age and chronological age (i.e. epigenetic age acceleration–EAA) has been informative of biological age in several conditions, including neurodegenerative diseases. To date, epigenetic clocks have not been evaluated in RBD.
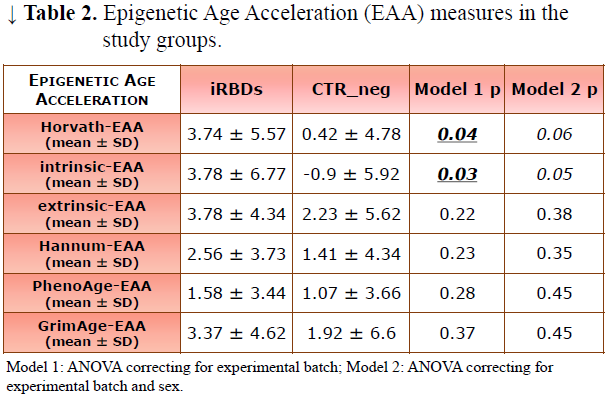
Method: We compared epigenetic age between vPSG-confirmed iRBDs (iRBD_pos) and vPSG-negative controls (CTR_vPSG). We considered several epigenetic clocks, based on distinct sets of CpG sites, which can be indicative of different aspects of biological age. We calculated EAA for each epigenetic age estimate, obtaining the following values: Horvath-EAA, Hannum-EAA, PhenoAge-EAA and GrimAge-EAA. We also calculated additional EAA measures derived from Horvath’s clock: intrinsic-EAA, independent from changes in blood cell composition, and extrinsic-EAA, indicative of immunosenescence.
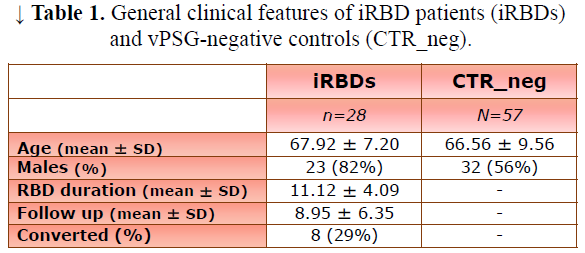
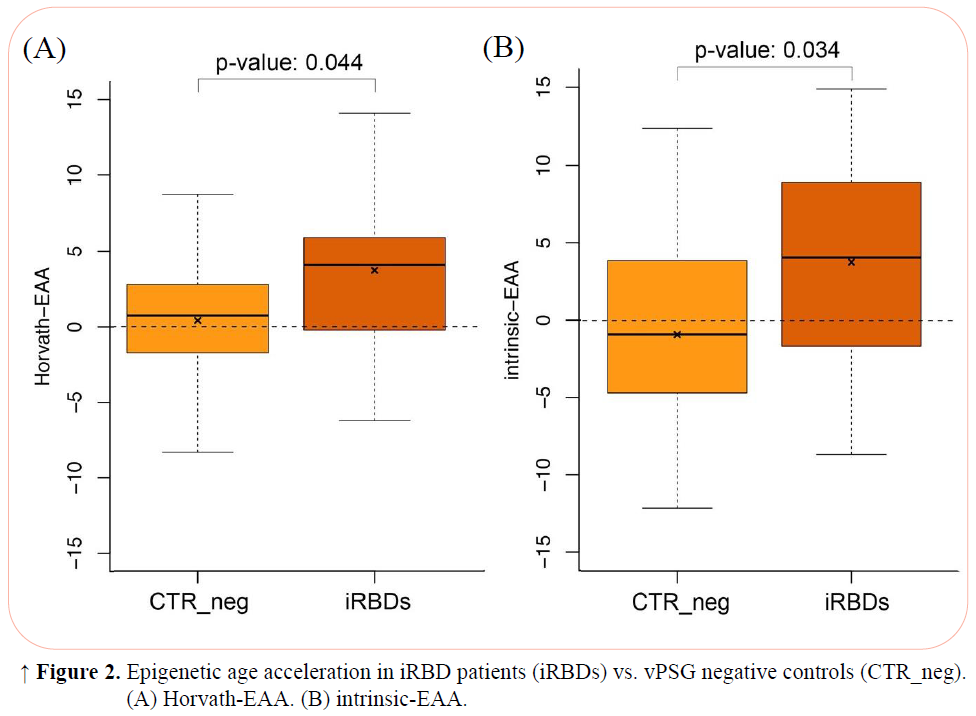
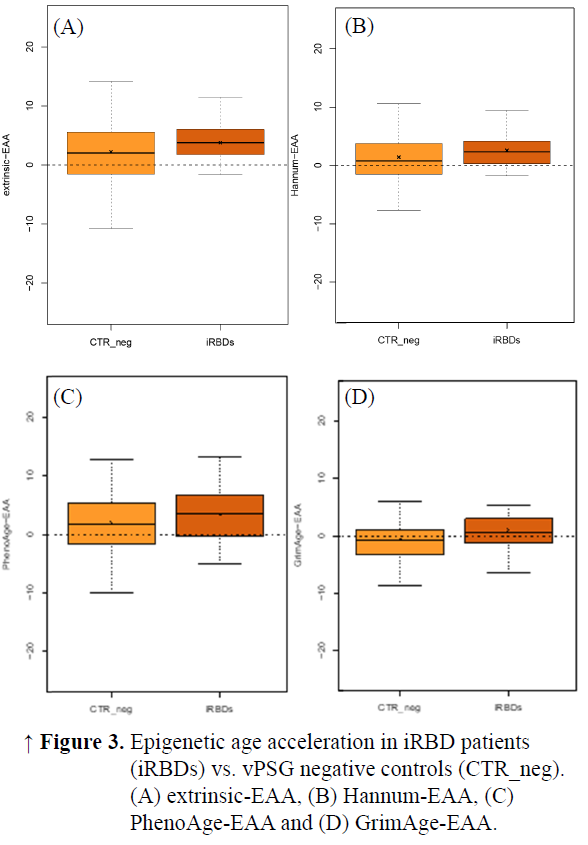
Results: We compared 28 iRBD_pos (23 males, age 67.92±7.20 years) and 57 CTR_vPSG (32 males, age 66.56±9.56 years) (Table 1). Compared to CTR_vPSG, iRBD_pos patients showed higher Horvath-EAA and intrinsic-EAA values when correcting for experimental batch (CTR_vPSG =0.42±4.78 iRBD_pos=3.74±5.57 p=0.04 and CTR_vPSG =-0.90±5.92 iRBD_pos=3.78±6.77 p=0.03 respectively). A similar trend was present also for most of the other EAA, without reaching statistical significance (Table 2–Model 1, Figures 1, 2). When sex was added as covariate, intrinsic-EAA values were confirmed as significantly different between CTR_vPSG and iRBD_pos (Table 2–Model 2).
Conclusion: Our results suggest the presence of an accelerated ageing process in iRBD patients in respect to controls. As previously demonstrated by Horvath and collaborators in Parkinson’s Disease, we suppose that the accelerated ageing in these patients reflects the neurodegenerative process already occurring. Bigger cohorts and longitudinal evaluations will allow us to strengthen these data and to evaluate their predictive value.
References: 1) Miglis, M. G. et al. Biomarkers of conversion to α-synucleinopathy in isolated rapid-eye-movement sleep behaviour disorder. Lancet. Neurol. 20, 671–684 (2021).
2) Franceschi, C. et al. The continuum of aging and age-related diseases: Common mechanisms but different rates. Front. Med. 5, (2018).
3) Horvath, S. & Ritz, B. R. Increased epigenetic age and granulocyte counts in the blood of Parkinson’s disease patients. Aging (Albany. NY). 7, 1130–1142 (2015).
To cite this abstract in AMA style:
L. Baldelli, C. Pirazzini, L. Sambati, F. Ravaioli, D. Gentilini, G. Calandra-Buonaura, P. Guaraldi, C. Franceschi, P. Cortelli, P. Garagnani, MG. Bacalini, F. Provini. Epigenetic clocks suggest accelerated ageing in isolated REM Sleep Behavior Disorder patients [abstract]. Mov Disord. 2023; 38 (suppl 1). https://www.mdsabstracts.org/abstract/epigenetic-clocks-suggest-accelerated-ageing-in-isolated-rem-sleep-behavior-disorder-patients/. Accessed February 11, 2026.« Back to 2023 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/epigenetic-clocks-suggest-accelerated-ageing-in-isolated-rem-sleep-behavior-disorder-patients/