Category: Parkinson’s Disease: Clinical Trials
Objective: To evaluate the effect of prasinezumab on motor progression and motor complications in early-stage Parkinson’s disease (PD) using an exploratory delayed-start analysis of Part 2 (Week 104) of the Phase II PASADENA study (NCT03100149).
Background: Prasinezumab is a humanised monoclonal antibody designed to target aggregated α-synuclein and slow PD progression. PASADENA Part 1 did not meet the primary endpoint (Movement Disorders Society–Unified Parkinson’s disease Rating Scale [MDS-UPDRS] sum of Parts I+II+III); however, differences in MDS-UPDRS Part III scores suggest less motor progression in prasinezumab-treated participants than in the placebo group.
Method: Participants with early-stage PD (diagnosis ≤2 years at screening; Hoehn & Yahr Stages I–II) were randomised to receive intravenous prasinezumab every 4 weeks (1500 mg or 4500 mg) for 104 weeks (early-start group, n=204), or placebo for 52 weeks followed by prasinezumab (1500 mg or 4500 mg) for 52 weeks (delayed-start group, n=105). Participants were included in the analysis regardless of change in symptomatic therapy. Motor progression and motor complications were defined, respectively, as ≥5-point increase in MDS-UPDRS Part III and reaching a score of ≥1-point in MDS-UPDRS Part IV and analysed as time-to-event (TTE) using Cox proportional hazards models.
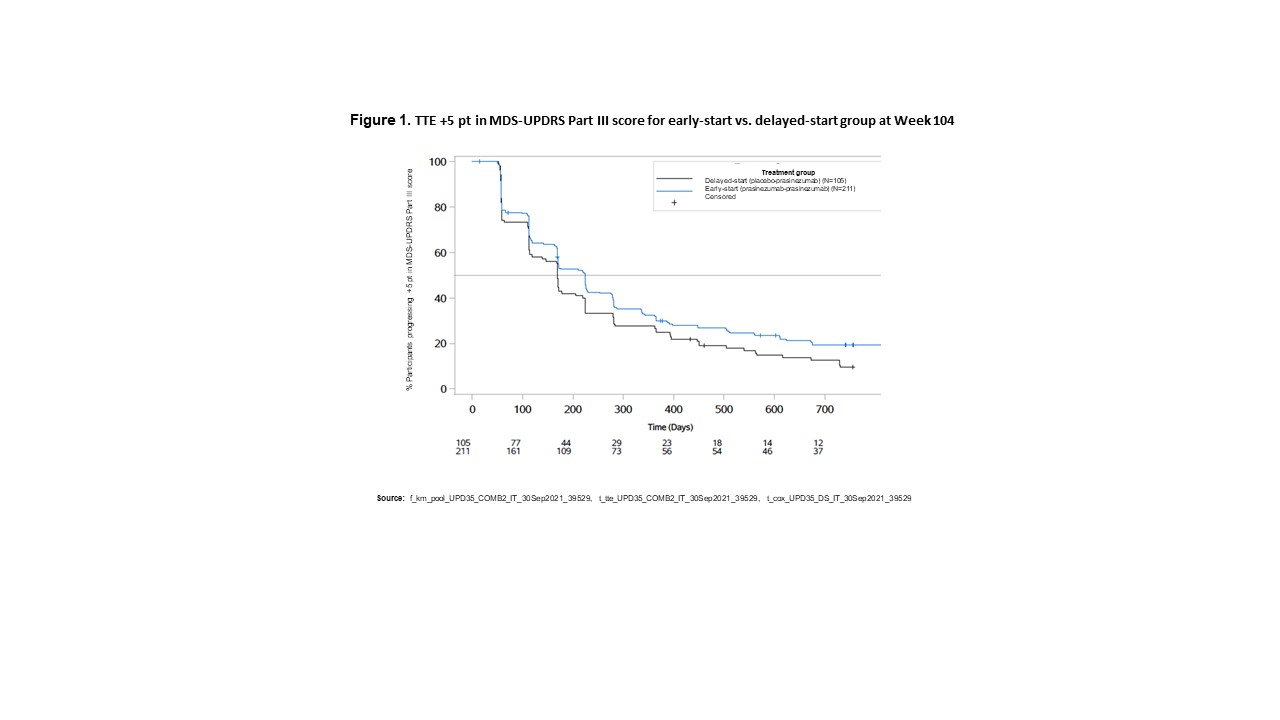
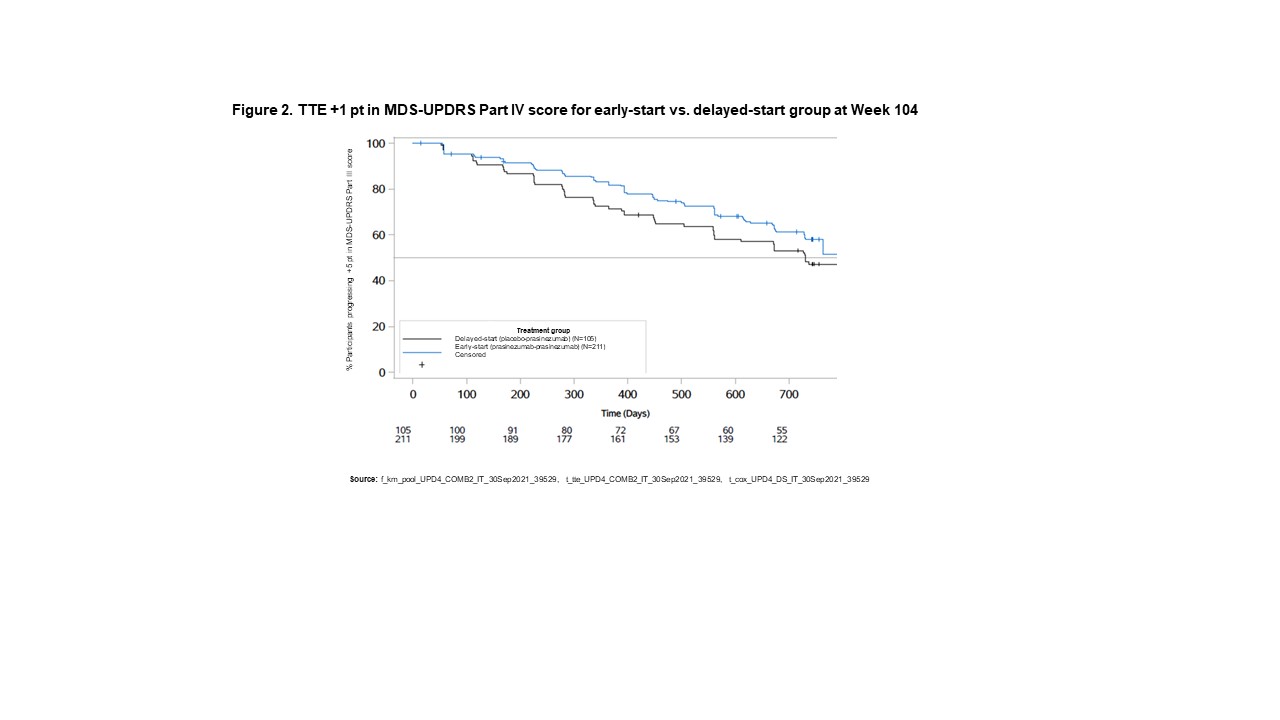
Results: Fewer participants in the early-start group (80.1%; median: 224 days) showed motor progression (reaching a ≥5-point increase in MDS-UPDRS Part III) (Figure 1) compared with the delayed-start group (89.5%; median: 169 days) (hazard ratio: 0.77 [80% CI 0.65–0.91]). Fewer participants in the early-start group (45.0%; median: 806 days) developed motor complications (reaching a score of ≥1-point in MDS-UPDRS Part IV) (Figure 2) compared with the delayed-start group (55.2%; median: 730 days) (hazard ratio: 0.74 [80% CI 0.60–0.92].
Conclusion: These findings suggest less motor progression and a lower risk of developing motor complications in early-stage PD participants treated with prasinezumab in the early-start group than in the delayed-start group.
To cite this abstract in AMA style:
G. Pagano, S. Zanigni, A. Monnet, K. Taylor, A. Hahn, T. Simuni, K. Marek, R. Postuma, N. Pavese, F. Stocchi, H. Svoboda, P. Fontoura, R. Doody, G. Kerchner, A. Bonni, T. Nikolcheva. Exploratory delayed-start analysis of PASADENA evaluating the efficacy of prasinezumab on motor progression and motor complications in early-stage Parkinson’s disease [abstract]. Mov Disord. 2022; 37 (suppl 2). https://www.mdsabstracts.org/abstract/exploratory-delayed-start-analysis-of-pasadena-evaluating-the-efficacy-of-prasinezumab-on-motor-progression-and-motor-complications-in-early-stage-parkinsons-disease/. Accessed July 18, 2025.« Back to 2022 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/exploratory-delayed-start-analysis-of-pasadena-evaluating-the-efficacy-of-prasinezumab-on-motor-progression-and-motor-complications-in-early-stage-parkinsons-disease/