Category: Spasticity
Objective: To evaluate goal attainment and ambulation in a subpopulation of TOWER study patients treated with UK licensed doses of incobotulinumtoxinA (incoA) for injection cycles 1 and 2; during cycle 3, dose could be uptitrated up to 800U (unlicensed).
Background: Increasing doses of incoA proved effective and well tolerated in patients with upper- and lower-limb spasticity due to cerebral lesions in the TOWER study.1
Method: TOWER was a prospective, multicenter trial of 155 patients with spasticity due to cerebral causes who received three escalating doses (400U, 600U, and up to 800U) incoA injected into upper and/or lower limbs. Goal attainment scale (GAS) ratings were measured at the end of each cycle and absolute and changes from study baseline in functional ambulation classification (FAC) scale scores at week 4 of cycles 1 and 2, and at the end of cycle 3.
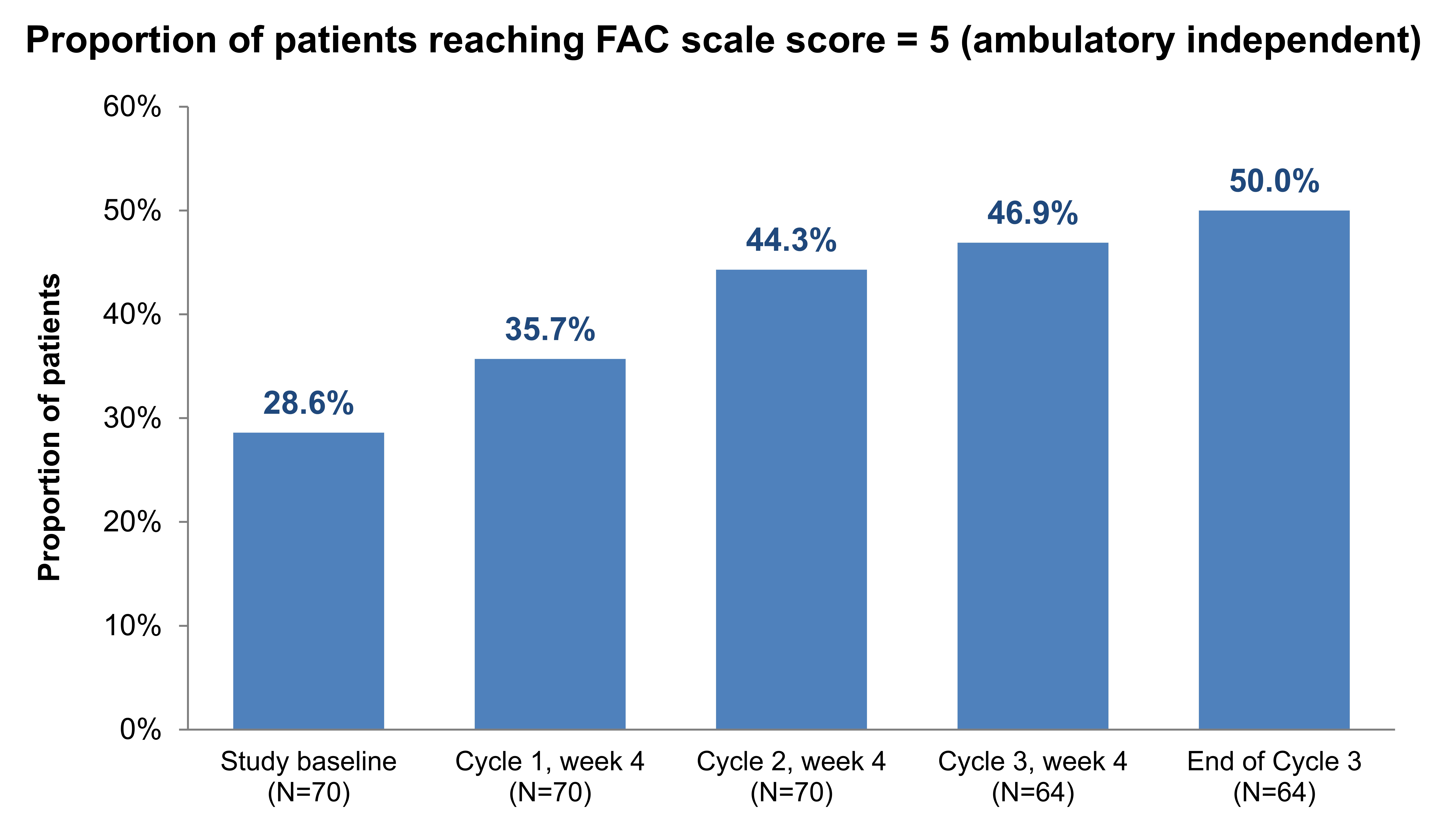
Results: This analysis included 70 patients (64.3% male; mean age 53.7 years) in whom stroke was the main cause of spasticity. Considering upper and lower limbs together, 25.7% (18/70) of patients achieved ≥3 (of 4 possible) treatment goals (GAS score ≥0) at the end of cycle 1 (400U incoA), compared with 51.4% (36/70) and 71.9% (46/64) at the end of cycle 2 (600U incoA) and cycle 3 (≤800U incoA), respectively. The proportion of ambulatory independent patients (FAC score=5) increased from 28.6% (20/70) at study baseline to 35.7% at week 4 in cycle 1, 44.3% at week 4 in cycle 2, 46.9% at week 4 of cycle 3, and 50% (32/64) at the end of cycle 3 (Figure).
A ≥1 point improvement in FAC scale score from baseline was achieved by 18.6% and 34.2% of 70 patients at week 4 of cycle 1 and cycle 2, respectively, and by 42.2% and 40.6% of 64 patients at week 4 of cycle 3 and by the end of cycle 3, respectively. IncoA proved well tolerated across all cycles. The rate of treatment-emergent adverse events (TEAEs) and serious TEAEs was 52.9% (N=37) and 8.6% (N=6), respectively. Across all incoA cycles, only 7.1% of patients (N=5) experienced any related TEAE.
Conclusion: As the number of injection cycles, dosage, and pattern treated with incoA increased, the tolerability of incoA was maintained and an increasing proportion of patients with upper and lower limb spasticity achieved more key treatment goals and were able to walk independently.
Previously presented at TOXINS 2024, 17-20 January.
Figure.
References: 1. Wissel J, et al. Safety and efficacy of incobotulinumtoxinA doses up to 800 U in limb spasticity. Neurology. 2017;88:1321–1328.
To cite this abstract in AMA style:
D. Bensmail, K. Fheodoroff, A. Hanschmann, L. Nicolson, W. Clarke, M. Vacchelli, J. Wissel. Goal Attainment and Ambulation With Increasing Doses of IncobotulinumtoxinA: A Post Hoc Analysis of the TOWER Study [abstract]. Mov Disord. 2024; 39 (suppl 1). https://www.mdsabstracts.org/abstract/goal-attainment-and-ambulation-with-increasing-doses-of-incobotulinumtoxina-a-post-hoc-analysis-of-the-tower-study/. Accessed July 9, 2025.« Back to 2024 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/goal-attainment-and-ambulation-with-increasing-doses-of-incobotulinumtoxina-a-post-hoc-analysis-of-the-tower-study/