Category: Parkinson’s Disease: Clinical Trials
Objective: To evaluate the effect of istradefylline (IST), an adenosine A2A receptor antagonist, on the adjustment of levodopa dose escalation in patients with Parkinson’s disease (PD) with wearing-off phenomena.
Background: Clinical data suggest using the lowest possible levodopa dose to balance treatment benefits against the risk of motor complications. IST is approved in Japan and the US as adjunctive treatment to levodopa/decarboxylase inhibitors in PD patients with wearing-off phenomena/off episodes. There is a lack of robust data on the impact of IST on levodopa dose titration.
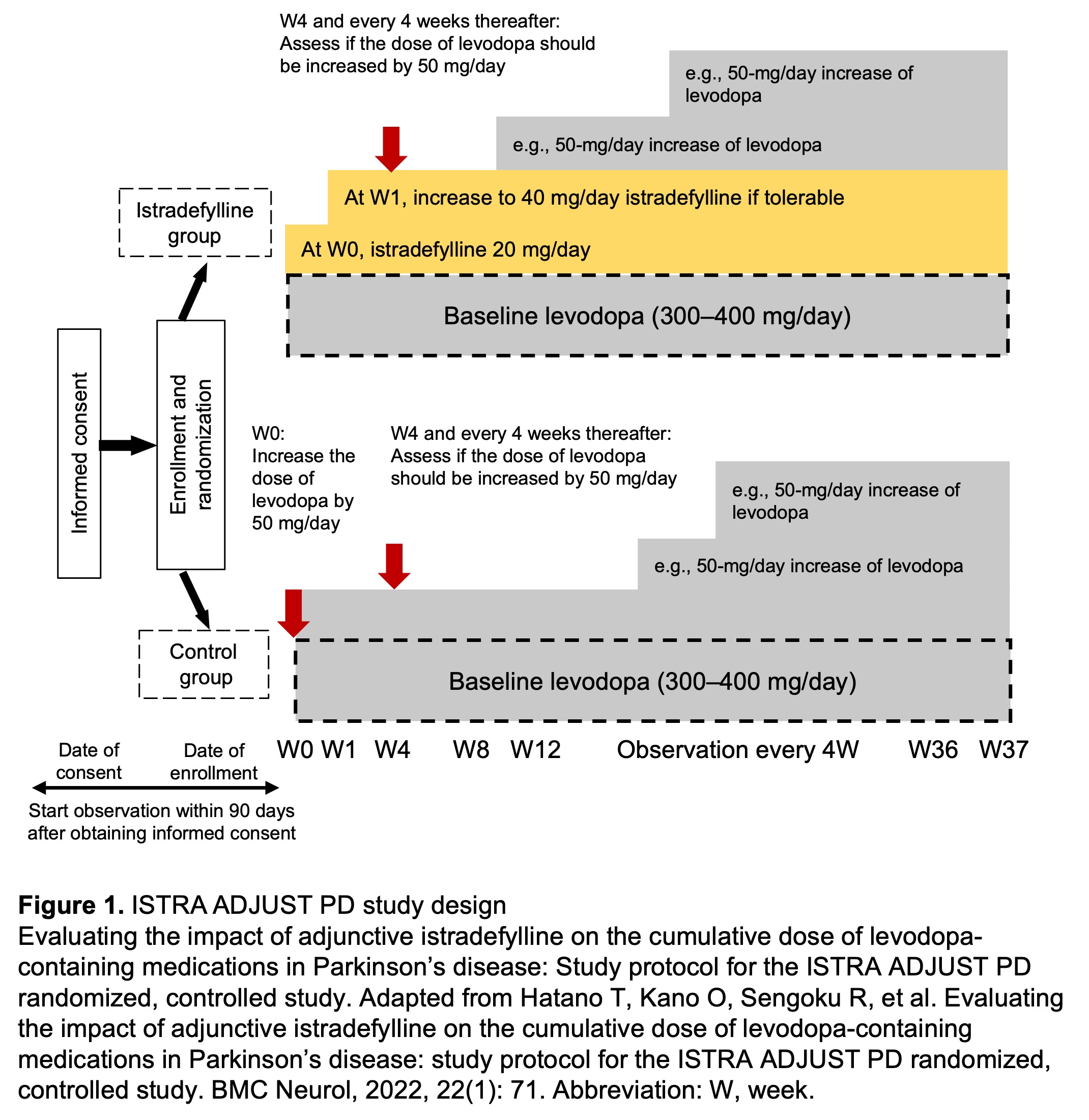
Method: In this multicenter, randomized, open-label, parallel-group controlled study, 114 PD patients with wearing-off phenomena receiving 300–400 mg/day levodopa were randomly assigned (1:1) to an IST or control group [figure1]. Levodopa dose escalation occurred based on clinical impression of severity. The primary endpoint was the cumulative additional levodopa dose throughout the 37-week (W) treatment period. Changes in symptom rating scales, motor activity using a wearable device, and safety outcomes were evaluated as secondary endpoints.
Results: In total, 105 patients (IST=52, control=53) were included in the efficacy and safety analyses. The cumulative additional levodopa dose as area under the curve was significantly lower by 5-fold in the IST vs. control group (p<0.0001, Mann–Whitney U test). Between 4W and 36W, the number of days to the first levodopa dose increase did not significantly differ between groups; 36.5% in the IST group and 47.2% in the control group had increased levodopa doses by 36W. Changes in secondary efficacy endpoints such as Movement Disorders Society-Unified Parkinson’s Disease Rating Scale from baseline were comparable between groups. Device-evaluated motor activities showed significant improvements from baseline to 36W only in the IST group. Incidences of adverse drug reactions (ADRs) were 28.8% in the IST group and 13.2% in the control group. No serious ADRs were observed in either group.
Conclusion: IST treatment was effective in the adjustment of levodopa dose escalation, resulting in less cumulative use of levodopa during the study. Data from the wearable device suggest that more objective improvement in motor function may be achieved with IST than with levodopa dose increase.
To cite this abstract in AMA style:
T. Hatano, O. Kano, R. Sengoku, N. Yanagisawa, H. Nagayama. Impact of istradefylline on the adjustment of levodopa dose escalation in Parkinson’s disease: Results of the ISTRA ADJUST PD randomized, controlled study [abstract]. Mov Disord. 2022; 37 (suppl 2). https://www.mdsabstracts.org/abstract/impact-of-istradefylline-on-the-adjustment-of-levodopa-dose-escalation-in-parkinsons-disease-results-of-the-istra-adjust-pd-randomized-controlled-study/. Accessed July 18, 2025.« Back to 2022 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/impact-of-istradefylline-on-the-adjustment-of-levodopa-dose-escalation-in-parkinsons-disease-results-of-the-istra-adjust-pd-randomized-controlled-study/