Category: Pediatric Movement Disorders
Objective: To present pooled data analyses of the immunogenicity of incobotulinumtoxinA (INCO) in pediatrics.
Background: BotulinumtoxinA (BoNT-A) is used to treat several chronic pediatric conditions, including spasticity and sialorrhea (excessive drooling). As with other biologic drugs, repeated use of BoNT-A can cause antibody (AB) formation leading to clinical non-responsiveness, a clinically challenging problem [1]. Because INCO, the most purified BoNT-A, is free from complexing proteins, it is thought to carry a low risk of inducing an immunogenic response [2].
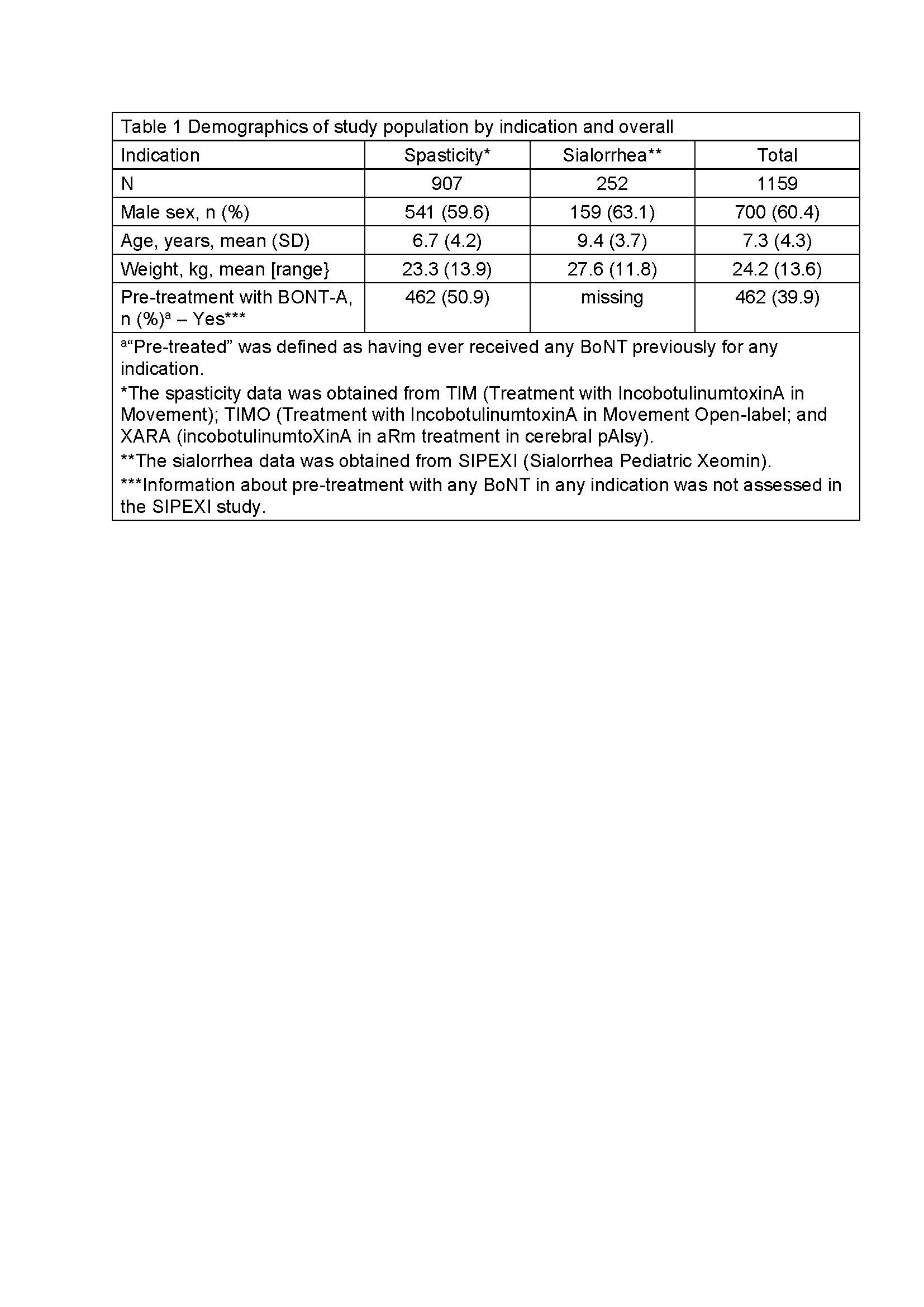
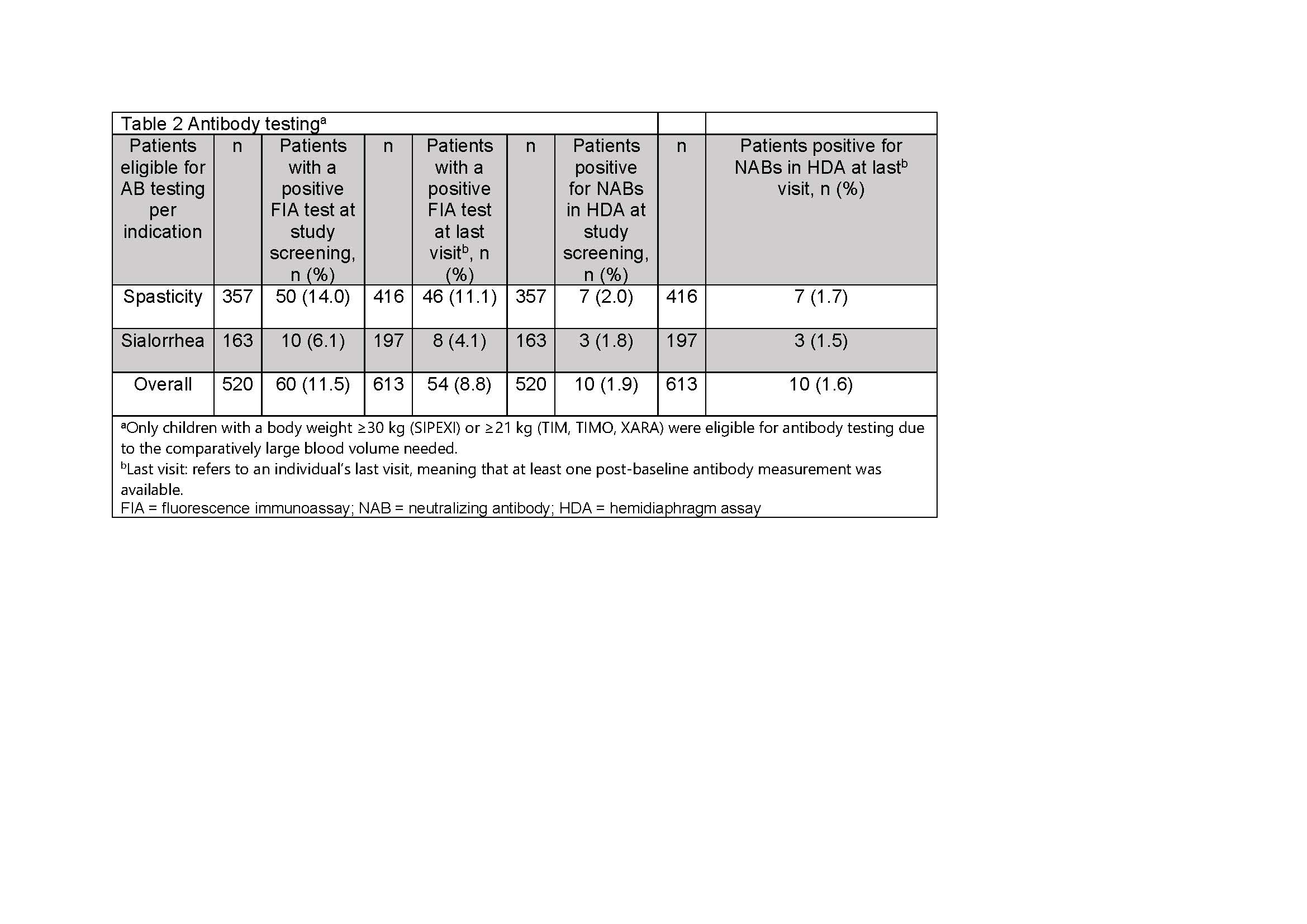
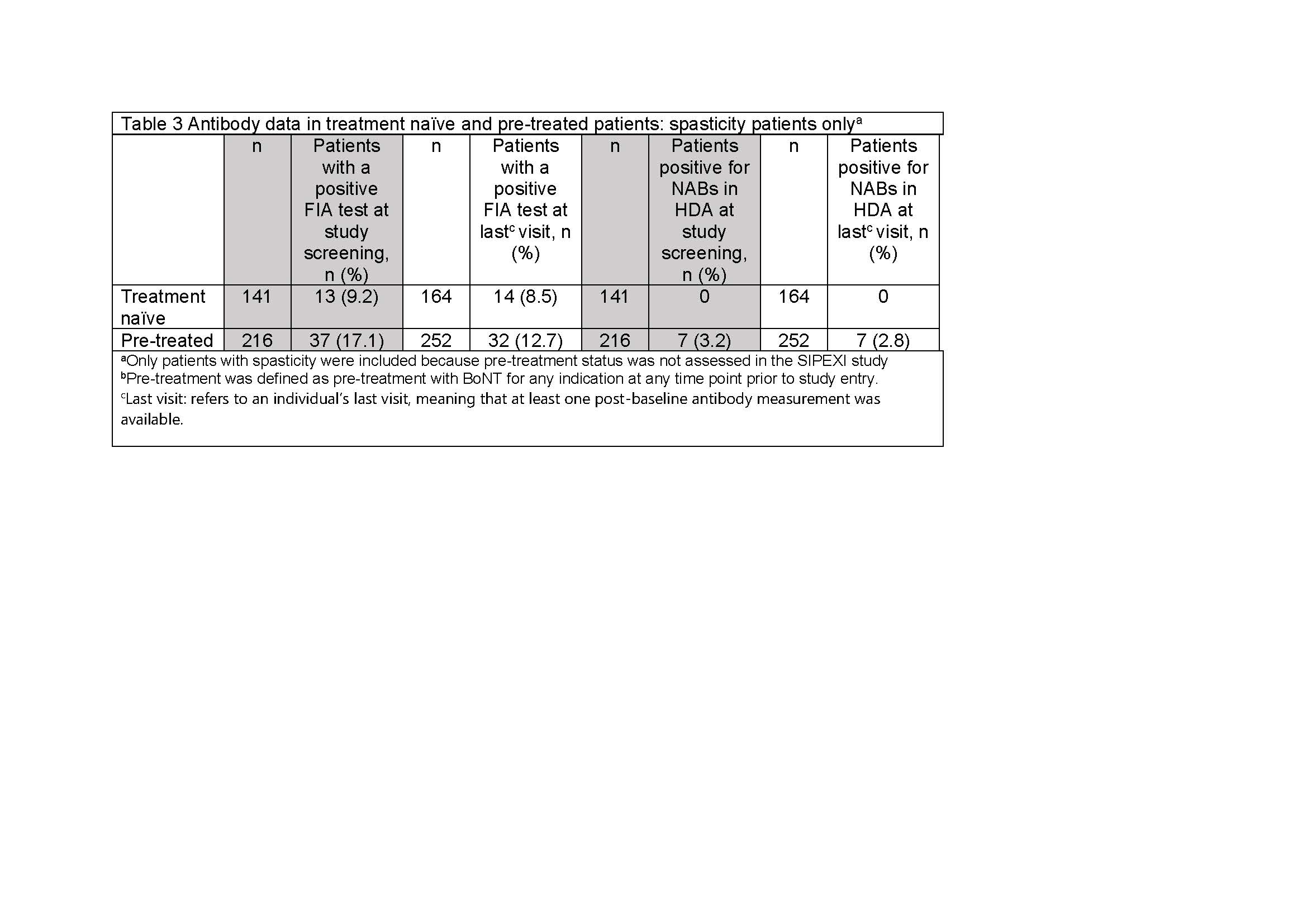
Method: This was a pooled analysis of available AB data from four phase 3 studies of children/adolescents (2–17 years) investigating INCO for the treatment of limb spasticity associated with cerebral palsy [Treatment with IncobotulinumtoxinA in Movement (TIM), Treatment with IncobotulinumtoxinA in Movement Open-label (TIMO), incobotulinumtoXinA in aRm treatment in cerebral pAlsy (XARA)] or sialorrhea [Sialorrhea Pediatric Xeomin Investigation (SIPEXI)] associated with neurological disorders in children/adolescents. Full methodological details can be found in [3–6]. Figure 1 [figure1] shows INCO doses, number/frequency of injection cycles (ICs), and timing of AB testing. Patient samples were first screened using fluorescence immunoassay (FIA) to detect antibodies against BoNT and, if positive, further testing for neutralizing (NAB) titers was performed using the mouse ex vivo hemidiaphragm assay (HDA). Data were pooled across INCO doses. AB testing was limited to patients ≥21 kg/≥30 kg body weight for the spasticity/sialorrhea studies, respectively.
Results: Of 1159 patients treated with INCO [Table 1], 520 were eligible for AB testing at screening and 422 (81.2%) test results were available; 60 (11.5%) had a positive FIA test at screening and 54 (8.8%) were positive at the last visit. Using the HDA, 10 (1.9%) tested positive for NABs at screening and 10 (1.6%) were NAB positive at the last visit [Table 2]. Analysis of the subpopulation with known pre-treatment status showed that no BoNT-naïve patients developed NABs after INCO; all children with positive NAB tests had previously been treated with a BoNT other than INCO [Table 3].
Conclusion: INCO carries a very low risk of inducing an immunogenic response with long-term use, and this can be an important determinant when selecting treatment for children/adolescents with chronic conditions.
References: 1. Carr WW, Jain N, Sublett JW. Immunogenicity of Botulinum Toxin Formulations: Potential Therapeutic Implications. Adv Ther. 2021;38(10):5046-64. doi: 10.1007/s12325-021-01882-9.
2. Kerscher M, Wanitphakdeedecha R, Trindade de Almeida A, Maas C, Frevert J. IncobotulinumtoxinA: A Highly Purified and Precisely Manufactured Botulinum Neurotoxin Type A. J Drugs Dermatol. 2019;18(1):52-7.
3. Heinen F, Kaňovský P, Schroeder AS, Chambers HG, Dabrowski E, Geister TL, et al. IncobotulinumtoxinA for the treatment of lower-limb spasticity in children and adolescents with cerebral palsy: A Phase 3 study. J Pediatr Rehabil Med, 2021; 14(2): 183-97. doi: 10.3233/PRM-210040.
4. Kaňovský P, Heinen F, Schroeder AS, Chambers HG, Dabrowski E, Geister TL, et al. Safety and efficacy of repeat long-term incobotulinumtoxinA treatment for lower limb or combined upper/lower limb spasticity in children with cerebral palsy. J Pediatr Rehabil Med (in press), 2021. doi: 10.3233/PRM-210041.
5. Dabrowski E, Chambers HG, Gaebler-Spira D, Banach M, Kaňovský P, Dersch H, et al. IncobotulinumtoxinA efficacy/safety in upper-limb spasticity in pediatric cerebral palsy: Randomized controlled trial. Pediatric Neurology 2021; 123: 10-20. doi: 10.1016/j.pediatrneurol.2021.05.014.
6. Berweck S, Bonikowski M, Kim H, Althaus M, Flatau-Baqué B, Mueller D, Banach MD. Placebo-Controlled Clinical Trial of IncobotulinumtoxinA for Sialorrhea in Children: SIPEXI. Neurology. 2021;97(14):e1425–36. doi: 10.1212/WNL.0000000000012573.
To cite this abstract in AMA style:
F. Heinen, M. Banach, E. Dabrowski, D. Gaebler-Spira, H. Chambers, S. Schroeder, T. Geister, M. Althaus, A. Hanschmann, S. Berweck. Lack of immunogenicity of incobotulinumtoxinA in pediatrics: A pooled analysis [abstract]. Mov Disord. 2022; 37 (suppl 2). https://www.mdsabstracts.org/abstract/lack-of-immunogenicity-of-incobotulinumtoxina-in-pediatrics-a-pooled-analysis/. Accessed July 10, 2025.« Back to 2022 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/lack-of-immunogenicity-of-incobotulinumtoxina-in-pediatrics-a-pooled-analysis/