Category: Parkinson’s Disease: Clinical Trials
Objective: To test safety and efficacy of the glucagon-like peptide 1 (GLP-1) agonist liraglutide in idiopathic Parkinson’s disease (PD).
Background: Insulin resistance (IR) may play a role in PD pathophysiology. GLP-1agonists, designedto improveIR, are currently under investigation as a treatment for PD. Liraglutide is a powerful GLP-1 agonist,used in diabetes and obesity treatment.
Method: In this single-center, randomized, double-blind, placebo-controlled trial, PD patients received self-administered liraglutide injections once-daily (1.2 or 1.8 mg, as tolerated) or placebo in a 2:1 study design for 52 weeks after titration. Primary outcomes includeda djusted difference in the OFF-state Movement Disorders Society Unified PD Rating Scale (MDS-UPDRS) part III, non-motor symptom scale (NMSS) and Mattis Dementia Rating Scale (MDRS-2) at week 54. Secondary outcomes included global MDS-UPDRS scores and subscores, quality of life scores (PDQ-39) and other neuropsychological tests. Efficacy analyses were based on an intention-to-treat design for patients completing post-randomization follow-up assessments.
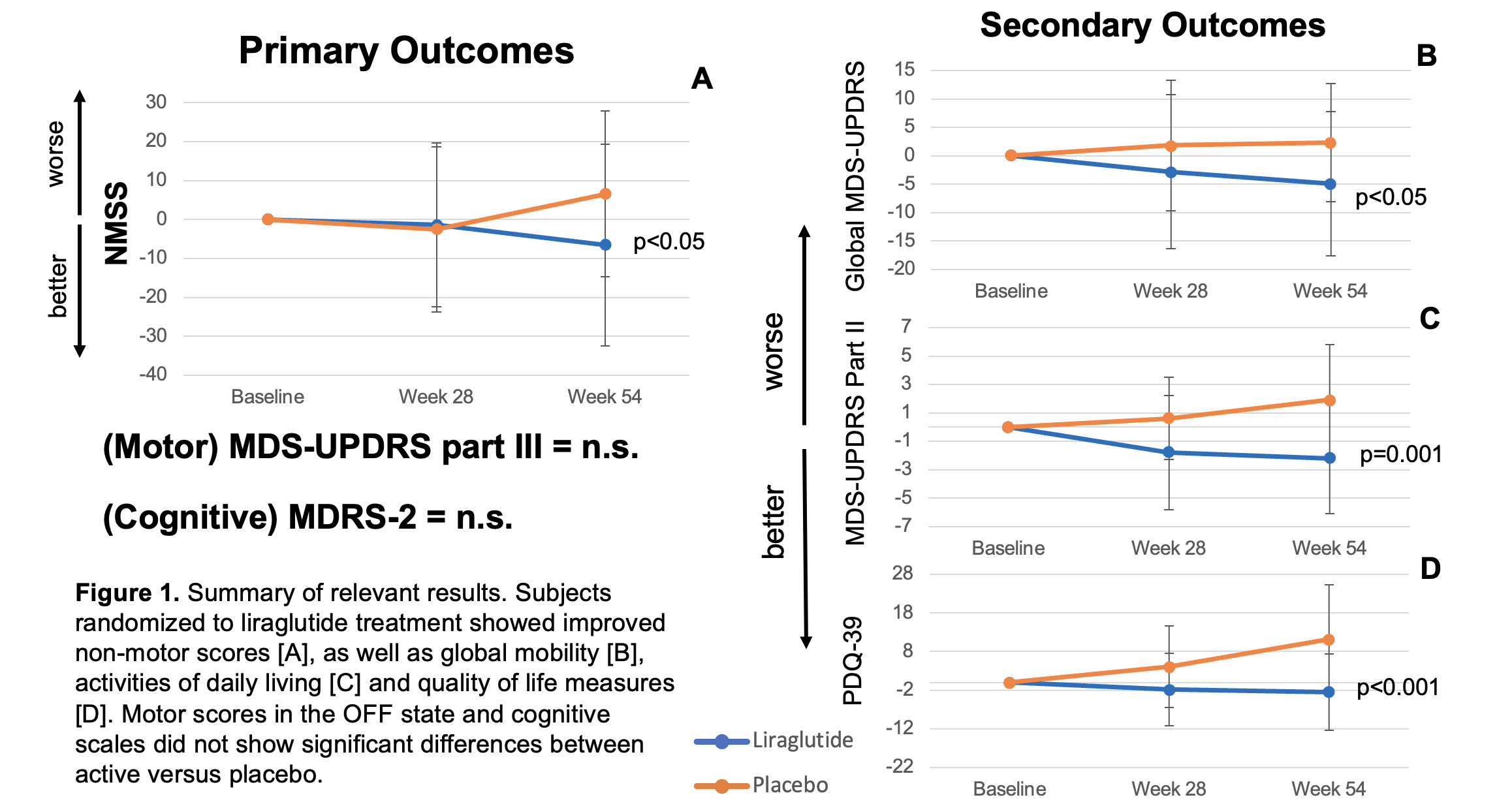
Results: 63 subjects were enrolled and randomized to liraglutide (n=42) or placebo (n=21). There were 12early withdrawals (9 liraglutide, 3 placebo), 4 of whom completed week 28 endpoint assessments. At 54 weeks, NMSS scores had improved by 6.6points in the liraglutide group and worsened by 6.5points in the placebo group, a 13.1point adjusted mean difference (p<0.05). MDS-UPDRS part III and MDRS-2 score changes did not significantly differ between liraglutide and placebo.Secondary outcome analyses revealed a significant improvement of global MDS-UPDRS (p<0.05), MDS-UPDRS part II (p=0.001), and PDQ-39 scores (p<0.001)in the treatment group. MADRS-2 subscores and other neuropsychological tests did not further differentiate study groups. Injection site reactions and gastrointestinal symptoms were common adverse events (AEs). 11serious AEs (9 liraglutide, 2 placebo) were reported, none related to the trial intervention.
Conclusion: Treatment with liraglutide is safe and improves critical features of PD, including non-motor symptoms, overall mobility, activities of daily living, and quality of life. These results validate similar outcomes reported with other GLP-1 agonists in PD and offer new strategies to treat the complexity of PD symptoms.
To cite this abstract in AMA style:
T. Wu, C. Bresee, J. Wertheimer, E. Hogg, C. Malatt, E. Tan, H. Pomeroy, G. Obialisi, M. Tagliati. Liraglutide once daily versus placebo in Parkinson’s disease: a randomized, double-blind, placebo-controlled trial [abstract]. Mov Disord. 2022; 37 (suppl 2). https://www.mdsabstracts.org/abstract/liraglutide-once-daily-versus-placebo-in-parkinsons-disease-a-randomized-double-blind-placebo-controlled-trial/. Accessed July 10, 2025.« Back to 2022 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/liraglutide-once-daily-versus-placebo-in-parkinsons-disease-a-randomized-double-blind-placebo-controlled-trial/