Category: Parkinson’s Disease: Clinical Trials
Objective: Evaluate long-term clinical outcomes and disease burden in people with advanced Parkinson’s disease (PwP) with motor fluctuations not optimized by their current medications.
Background: In advanced Parkinson’s disease (PD), uncontrolled motor fluctuations can impair quality of life. Real-world prospective data is needed on the evolution of disease and clinical outcomes in PwP with motor fluctuations not optimized by their current treatment regimen.
Method: PROSPECT was a 24-month international, prospective, observational study of adults (≥30 years) with idiopathic PD with insufficiently controlled motor fluctuations (“Off” time ≥ 2.5 hours per day). Participants must have had adequate trials of oral PD medications and could not have received device-aided treatment (DAT) prior to enrollment. The primary endpoint was the “Off” time change from baseline to month 24, assessed by PD diaries. Secondary endpoints included the change from baseline in Hoehn & Yahr (HY) stage for disease severity, MDS-UPDRS part II for activities of daily living, Parkinson’s Disease Questionnaire (PDQ-39) for quality of life, and non-motor symptom scale (NMSS). This analysis included participants who exclusively remained on oral PD medications during the study.
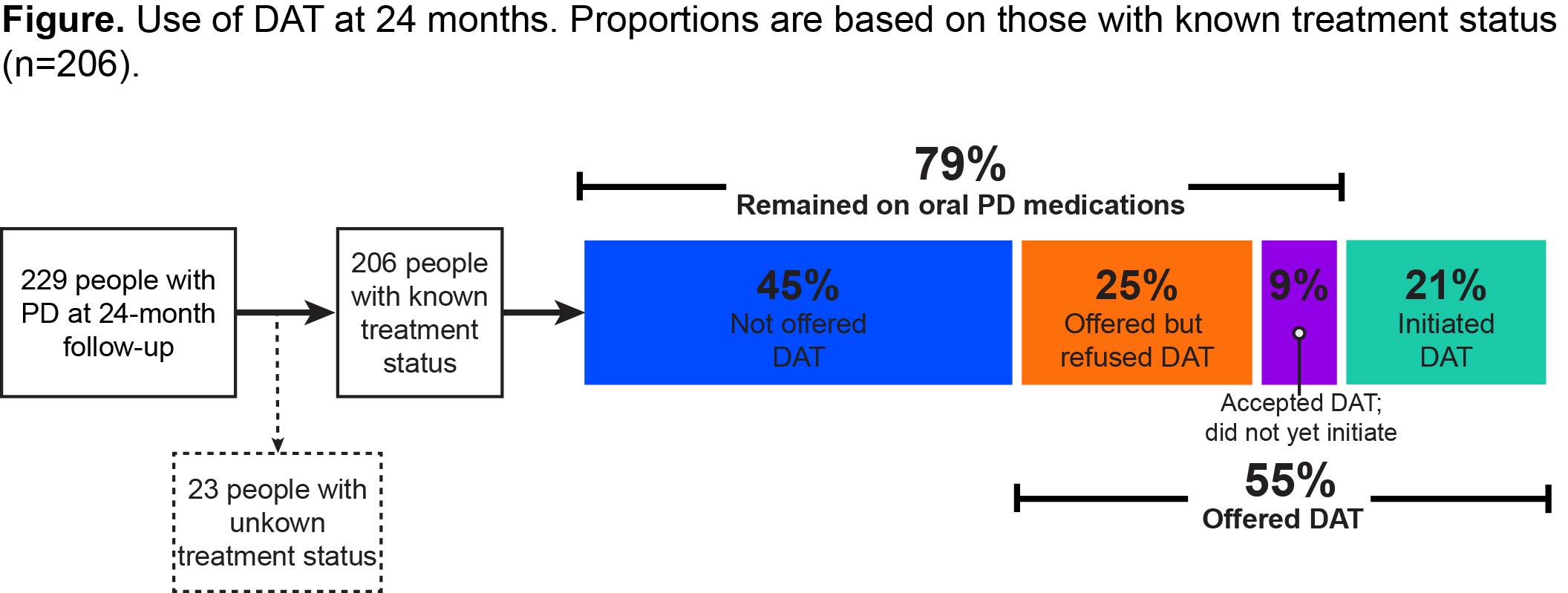
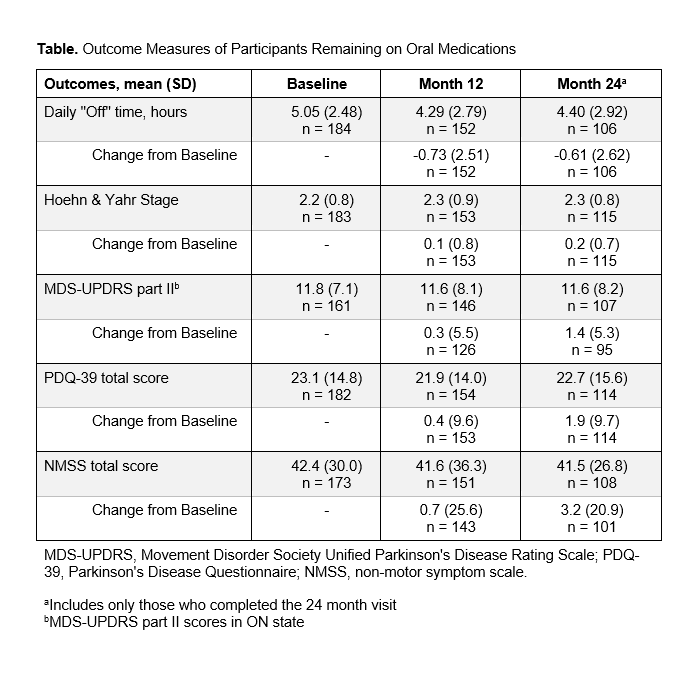
Results: The study included 229 PwP with a mean (SD) age of 68.4(9.7) years, mean (SD) disease duration of 9.0(5.5) years, and mean (SD) “Off” time of 4.99(2.45) hours at baseline. Of 206 PwP with known treatment status, 55%(n=113/206) were offered DAT at different points, but most (79%, n=162/206) remained exclusively on oral PD medications during the follow-up period [Figure]. Those exclusively on oral PD medications showed minimal, not clinically relevant changes in daily “Off” time from baseline to 24 months (mean [SD] -0.61[2.62] hours [Table]). No remarkable changes were observed at 24 months in HY, MDS-UPDRS part II, PDQ-39, and NMSS scores (mean [SD] change from baseline 0.2[0.7], 1.4[5.3], 1.9[9.7], and 3.2[20.9], respectively).
Conclusion: In this 24-month observational study, most PwP stayed on oral PD medications despite eligibility for DAT. Motor symptoms remained uncontrolled, and there were no improvements in disease burden despite care from a movement disorder specialist or clinic. Further comparison to those who initiated DAT will provide insight into the impact of timely advanced therapy use on clinical outcomes.
Figure
Table
To cite this abstract in AMA style:
A. Espay, H. Watanabe, A. Lehn, F. Ory-Magne, T. Mestre, D. Safarpour, L. Bergmann, P. Kukreja, K. Onuk, P. Alonso. Long-term evaluation of advanced Parkinson’s disease burden and clinical outcomes: results from the PROSPECT observational study [abstract]. Mov Disord. 2024; 39 (suppl 1). https://www.mdsabstracts.org/abstract/long-term-evaluation-of-advanced-parkinsons-disease-burden-and-clinical-outcomes-results-from-the-prospect-observational-study/. Accessed July 3, 2025.« Back to 2024 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/long-term-evaluation-of-advanced-parkinsons-disease-burden-and-clinical-outcomes-results-from-the-prospect-observational-study/