Objective: We focus on the novel endogenous neural repair function that comes from the mitochondrial transfer, and we aim to elucidate the molecular mechanism by which the LRRK2 G2019S mutation participates in the pathogenesis of PD through the mitochondrial transfer-dependent pathway.
Background: The LRRK2 G2019S mutation is one of the most common genetic risks for PD, but its pathogenic mechanism is unclear. Our previous research reported that astrocytes induced by hiPSCs deliver healthy mitochondria to DA neurons in the PD model and exert neuroprotective effects. It suggests that intercellular mitochondrial transfer may be a endogenous neural repair mechanism. We then design and experiment to determine whether the LRRK2 G2019S mutant damages endogenous neural repair and leads to PD by mitochondrial transfer-dependent pathways.
Method: Cell reprogramming technology, Establishing an “astrocyte-DA neuron” co-culture system. live cell staining, fluorescence confocal technology, Co-IP, and WB.
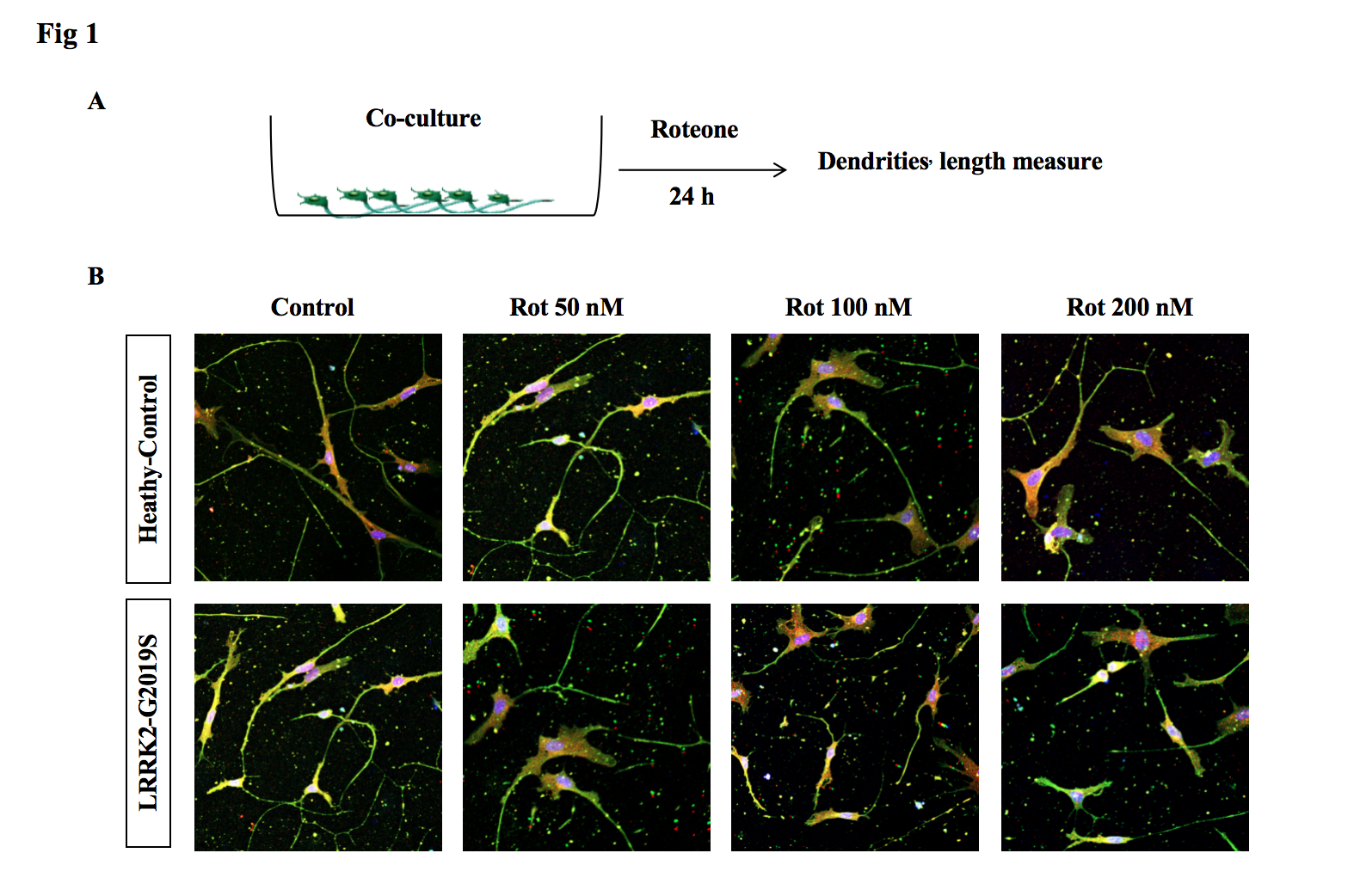
Results: 1, In the co-culture system carrying LRRK2 G2019S mutation, the loss of DA neurons and shortened of axons is more significant.
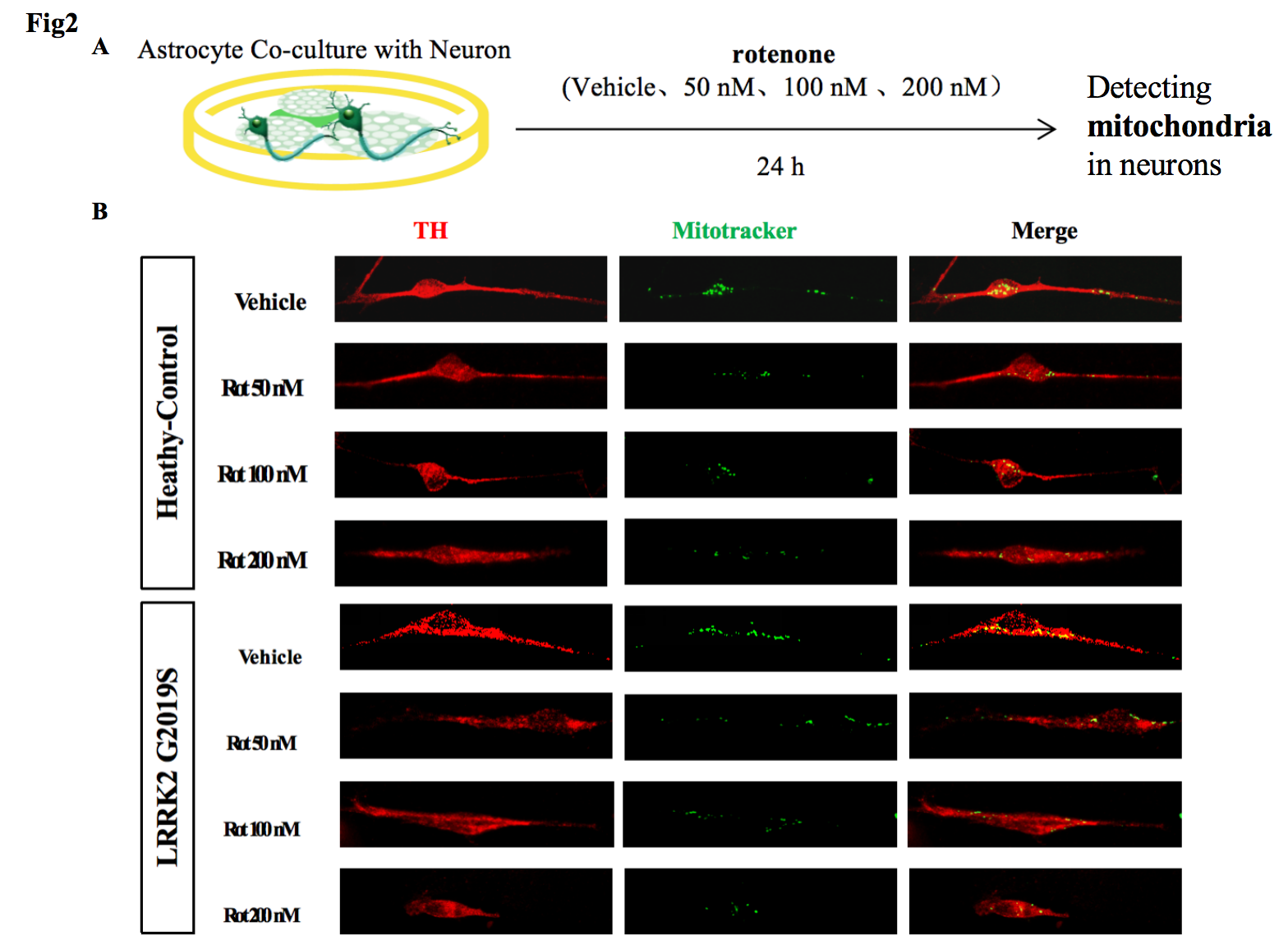
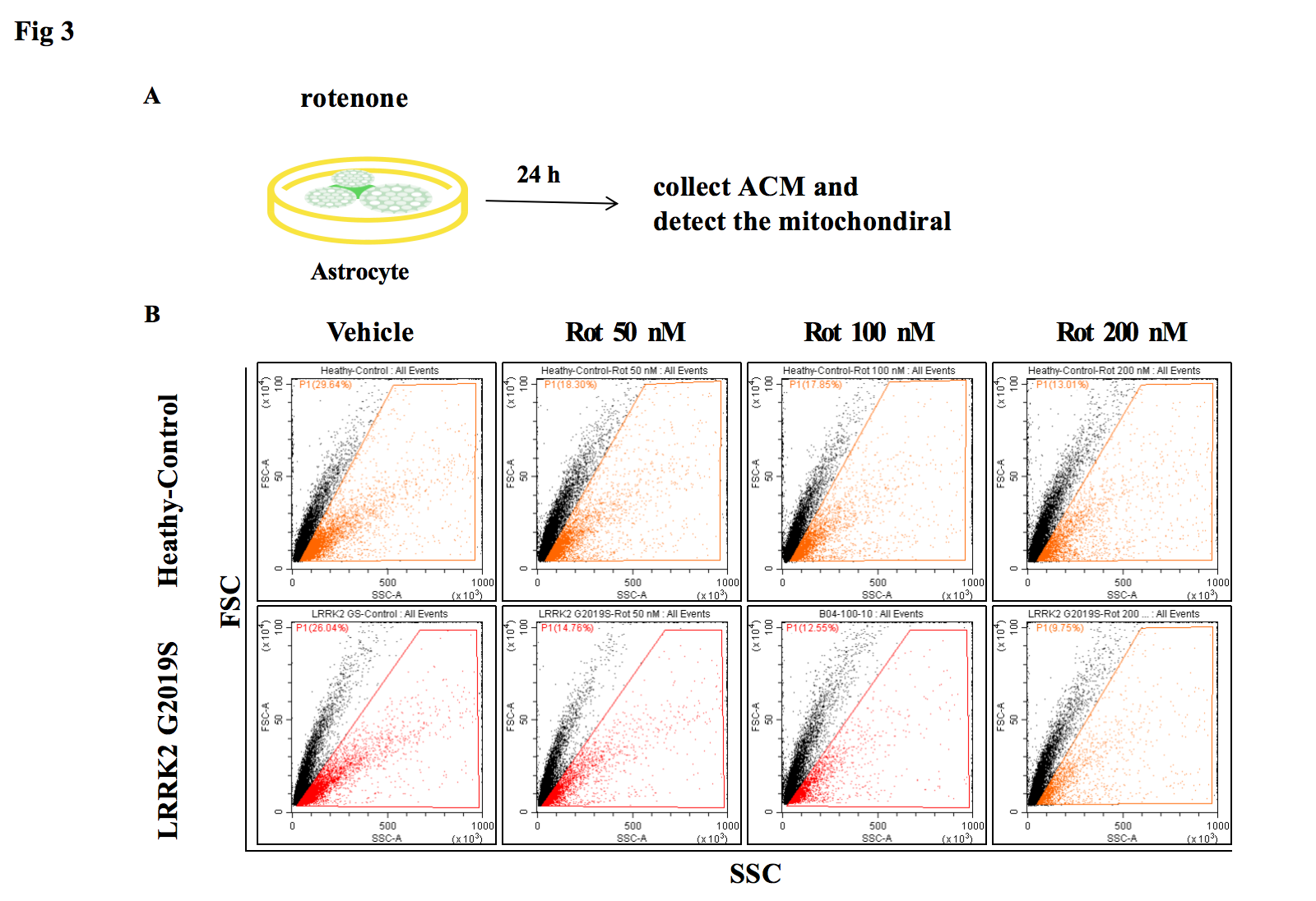
2, In the co-culture system carrying LRRK2 G2019S mutation, mitochondrial transfer is reduced more significantly.
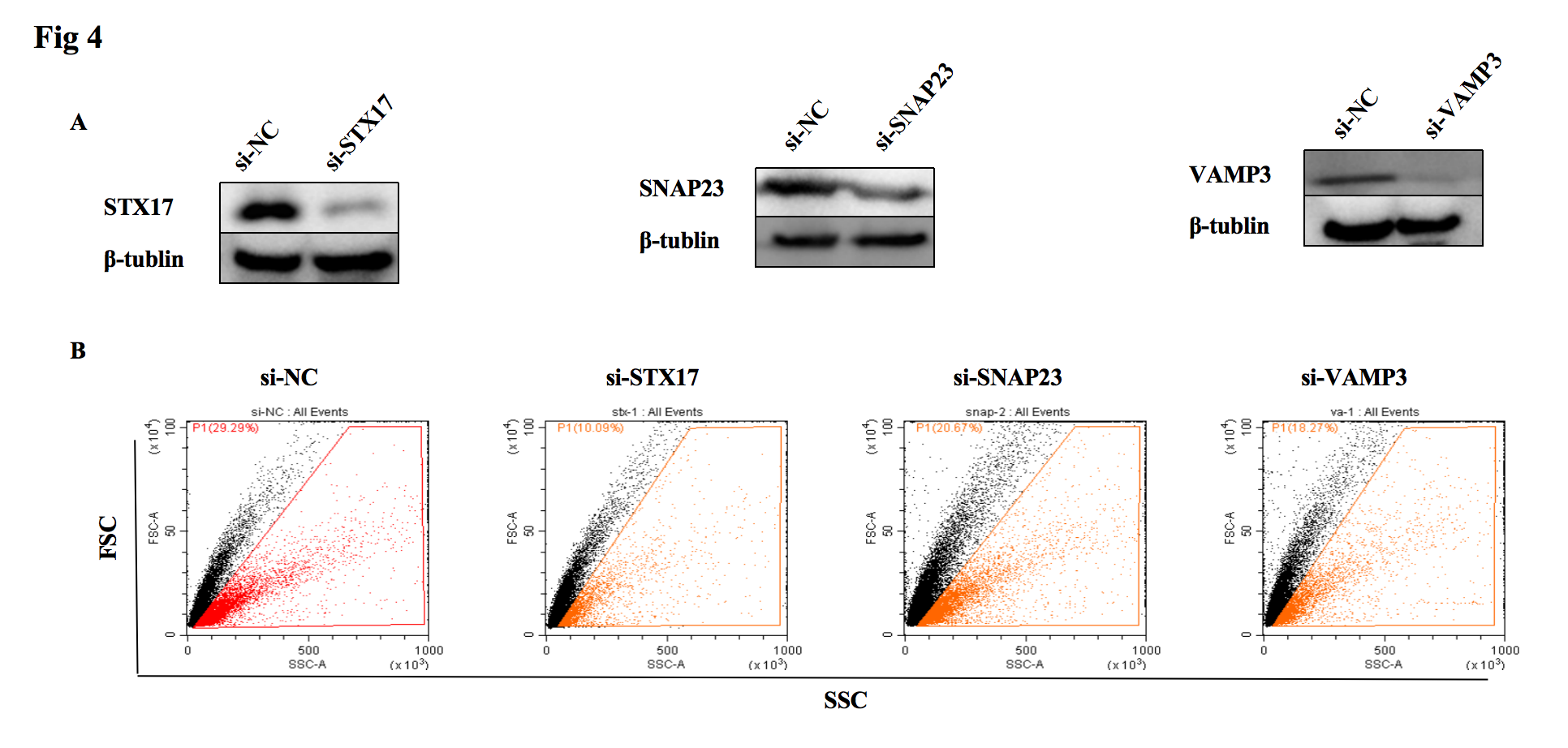
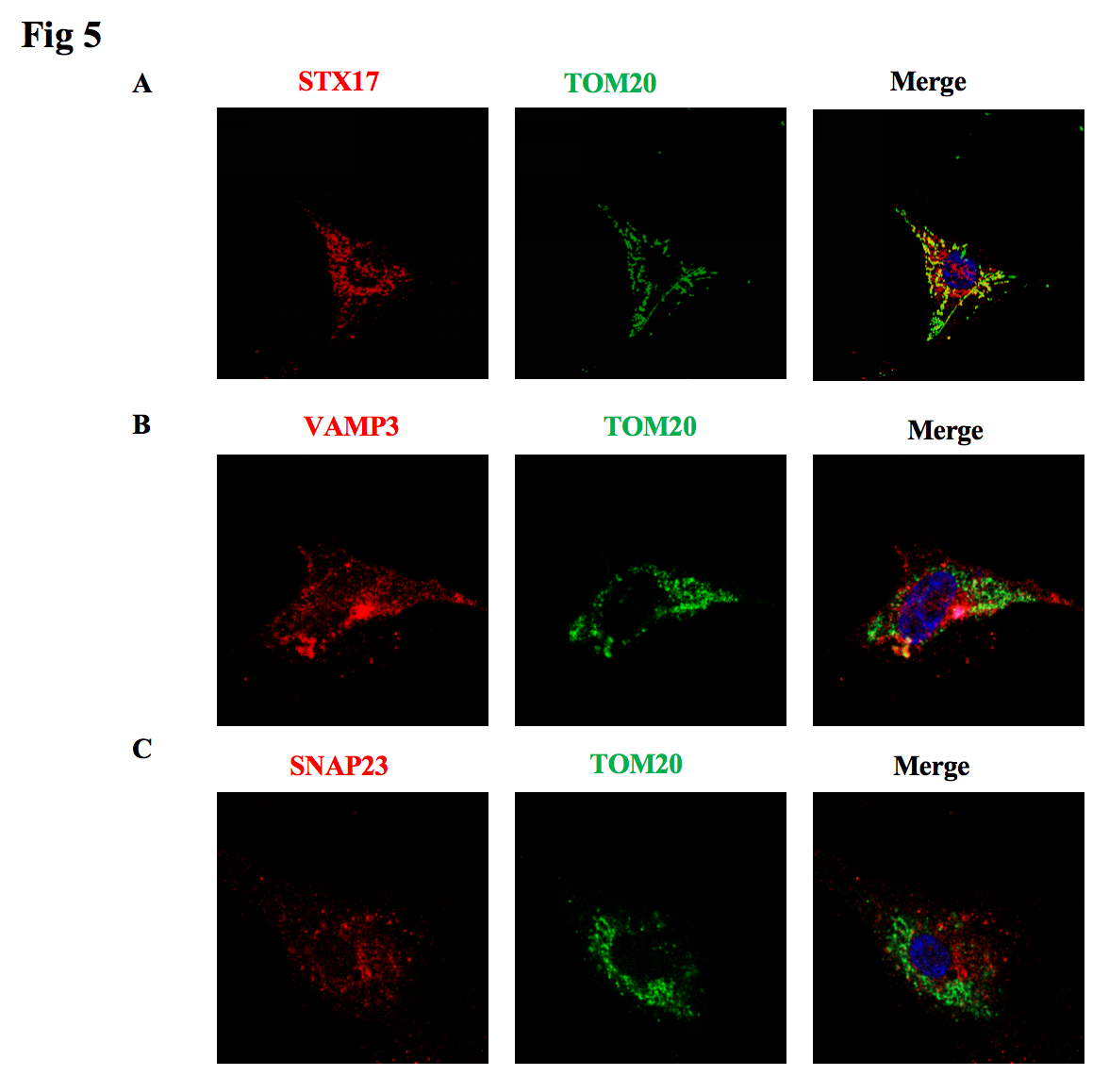
3, Knockdown of the SNARE complex members STX17, SNP23, and VAMP3 results in a decrease in mitochondrial output from astrocytes, particularly STX17.
4, STX17 is co localized with the mitochondrial outer membrane, while SNP23, VAMP3 are not co localized with the mitochondrial outer membrane.
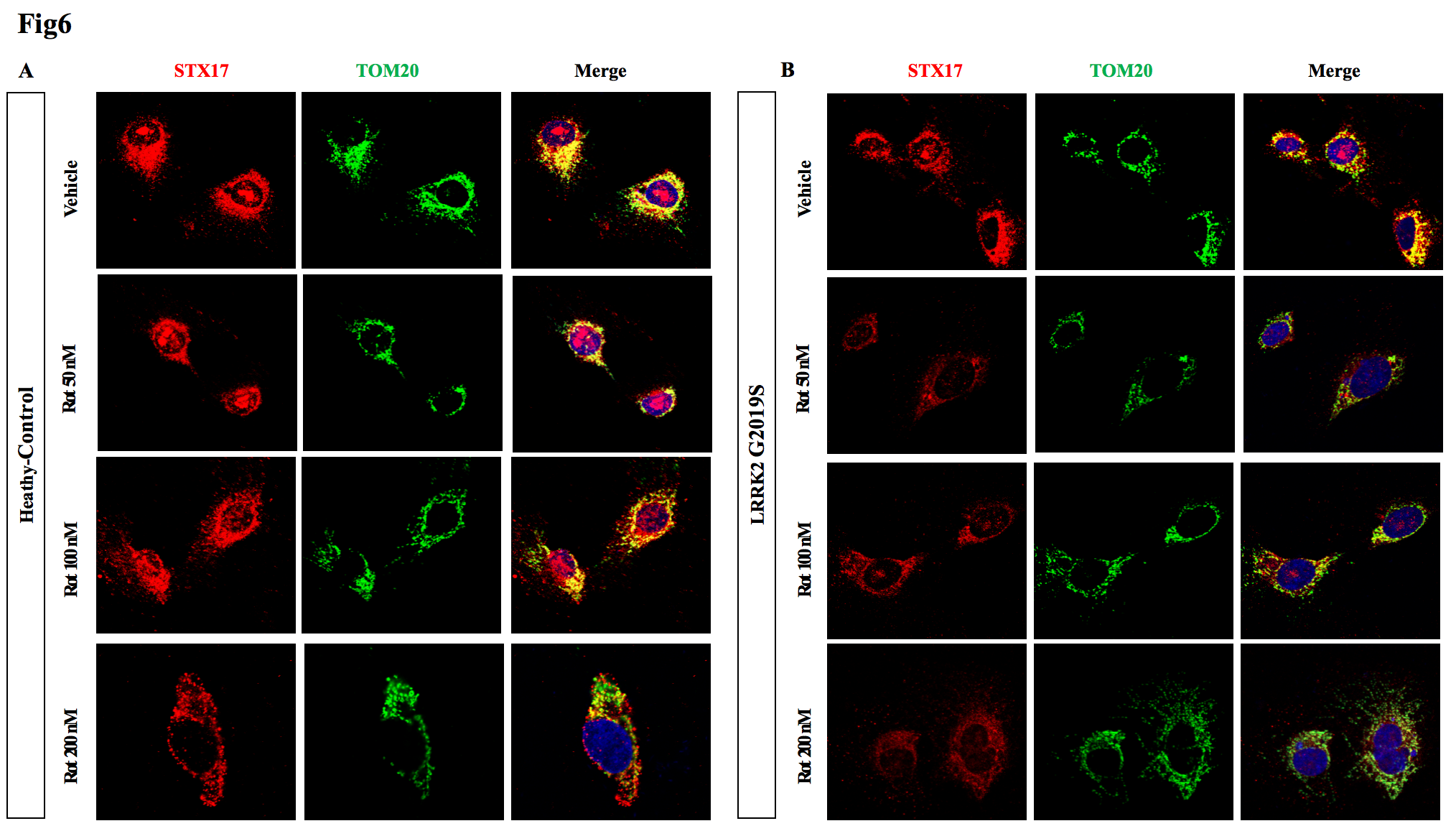
5, After exposure to rotenone, the co-localization of STX17 with mitochondria decreased, especially in the astrocytes carrying LRRK2 G2019S mutant.
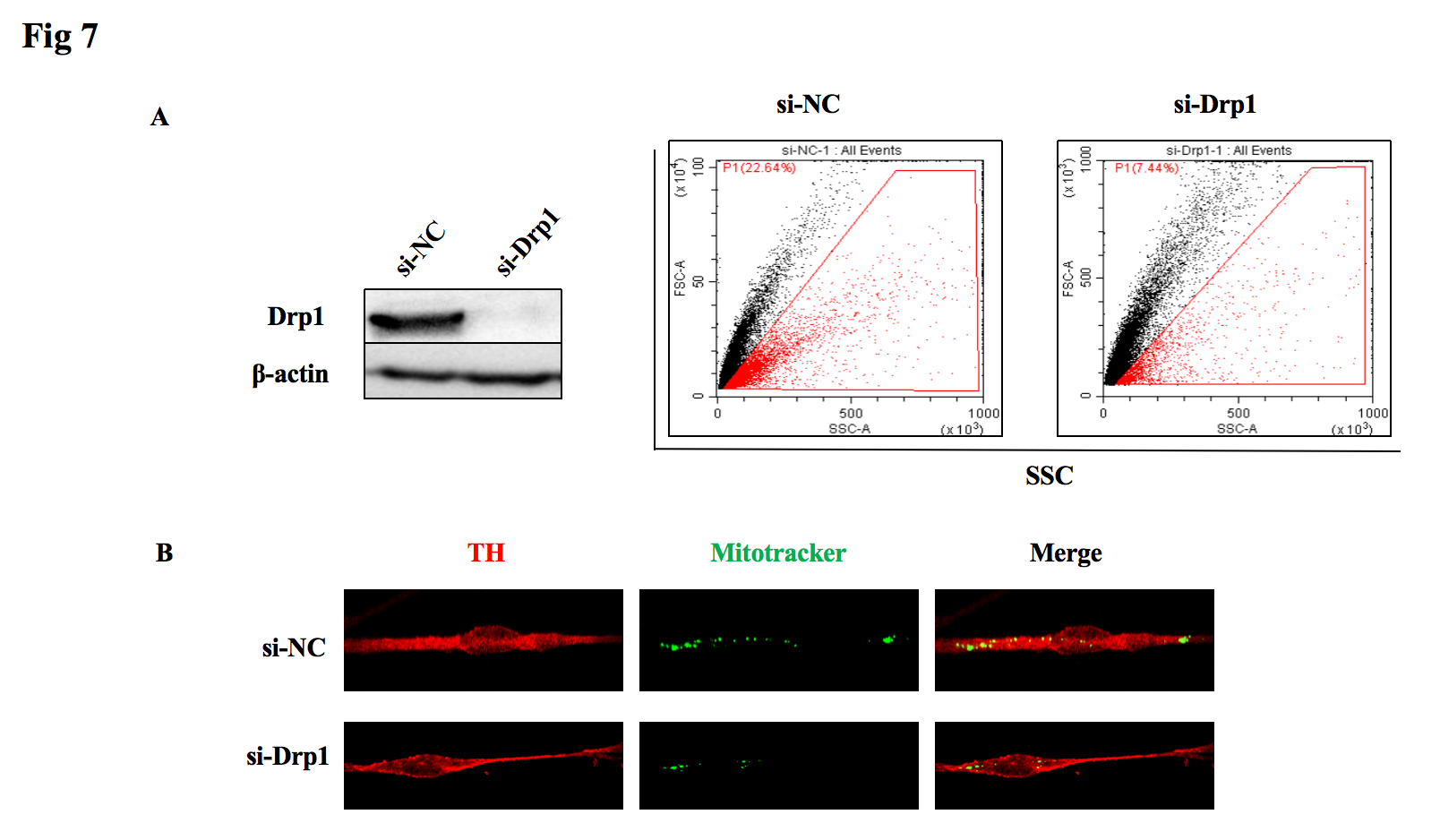
6, Knockdown of mitochondrial dynamic-related protein Drp1 results in a decrease in mitochondrial output.
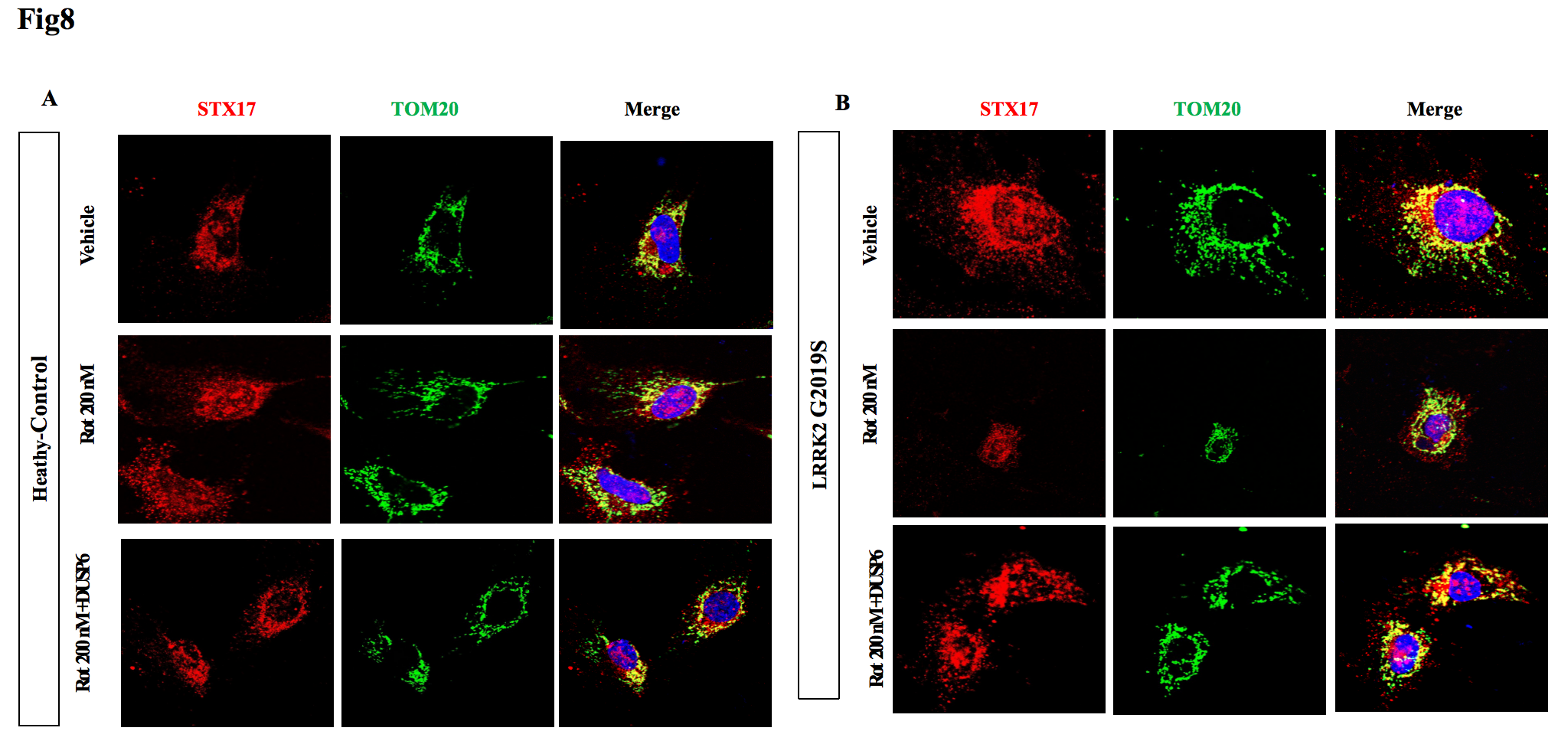
7, Drp1 phosphorylation inhibitor treatment leads to an increased co-localization of STX17 and mitochondria.
Conclusion: LRRK2 G2019S mutation damages the endogenous neural repair mechanism mediated by mitochondrial transfer. STX17 is an important protein for mitochondrial transfer. The forming SNAREs complexes do not participate in the exocytosis of mitochondria, but rather the mitochondrial dynamic key protein Drp1 might regulate mitochondrial transfer. LRRK2 G2019S mutation causes excessive phosphorylation of Drp1, which prevents the interaction of STX17 and mitochondria, which might be an key pathogenic link.
Fig1
Fig2
Fig3
Fig4
Fig5
Fig6
Fig7
Fig8
To cite this abstract in AMA style:
X. Cheng. LRRK2 G2019S mutant damages mitochondrial transfer by a Drp1-STX17 depend pathway in Parkinson’s disease [abstract]. Mov Disord. 2024; 39 (suppl 1). https://www.mdsabstracts.org/abstract/lrrk2-g2019s-mutant-damages-mitochondrial-transfer-by-a-drp1-stx17-depend-pathway-in-parkinsons-disease/. Accessed March 7, 2026.« Back to 2024 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/lrrk2-g2019s-mutant-damages-mitochondrial-transfer-by-a-drp1-stx17-depend-pathway-in-parkinsons-disease/