Objective: To describe the effects of bemdaneprocel on motor and non-motor outcomes in participants with Parkinson’s disease (PD) up to 24 months post transplantation (12 months post discontinuation of immunosuppression).
Background: Bemdaneprocel is an investigational therapy comprising embryonic stem cell-derived dopaminergic neuronal progenitors; proof of concept and safety are supported by preclinical studies. At 12 months post transplantation, predefined safety, tolerability, and feasibility criteria were met, with early indications of possible clinical benefit.
Method: In this phase 1, open-label, 24-month, non-controlled study (exPDite, NCT04802733), 12 participants with PD received a low dose (n=5; 0.9 million cells/putamen) or high dose (n=7; 2.7 million cells/putamen) of bemdaneprocel injected bilaterally into the postcommissural putamen with a cannula in a single surgical session. A 12-month immunosuppression regimen began intraoperatively. Changes from baseline at 18 months post transplantation in motor and non-motor outcomes are reported.
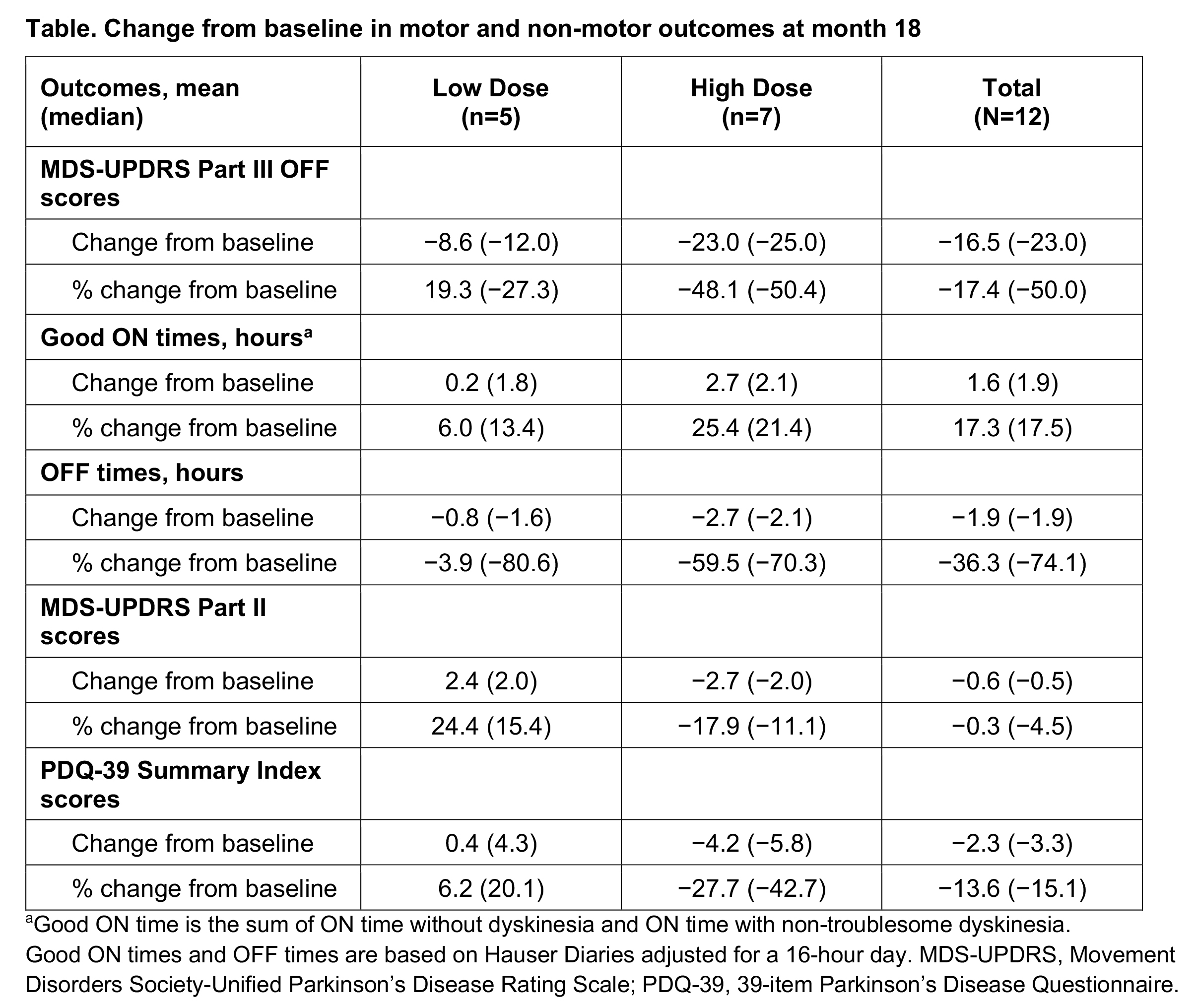
Results: Participants (N=12) were median 67.0 years of age, 75% male, and 67% White; median (Q1, Q3) time since PD diagnosis was 9.0 years (5.9, 11.5). In the high-dose cohort at 18 months post transplantation, mean (standard deviation [SD]) changes from baseline in MDS-UPDRS Part III OFF scores (−23.0 points [7.9]) and patient-reported Good ON times (2.7 hours [1.6]) and OFF times (−2.7 hours [1.8]) indicated a continuing trend toward clinical benefit [Table]. MDS-UPDRS Part II and PDQ-39 summary index scores changed by mean (SD) −2.7 points (5.2) and −4.2 points (8.2), respectively, in the high-dose cohort. Results in the low-dose cohort indicated stability or mild improvement.
Conclusion: Overall, trends toward benefit in motor symptoms and stability in non-motor symptoms were reported for participants who received bemdaneprocel. These effects persisted for 6 months post discontinuation of immunosuppression. All participants are expected to be followed in a long-term extension study (NCT05897957). These results support the continued development and evaluation of bemdaneprocel for the treatment of people with PD. New results from exPDite, 24 months post transplantation (12 months post discontinuation of immunosuppression), will be presented at the congress.
Table
References: 1. Piao J, Zabierowski S, Dubose BN, et al. Preclinical efficacy and safety of a human embryonic stem cell-derived midbrain dopamine progenitor product, MSK-DA01. Cell Stem Cell. 2021;28(2):217–229.
2. Kriks S, Shim JW, Piao J, et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature. 2011;480(7378):547–551.
3. Henchcliffe C, Sarva H, Lozano A, et al. Dopaminergic neuronal cell therapy for Parkinson’s disease: results from a Phase 1 study of bemdaneprocel [poster]. Presented at: International Congress of Parkinson’s Disease and Movement Disorders; August 27–31, 2023; Copenhagen, Denmark.
To cite this abstract in AMA style:
C. Henchcliffe, H. Sarva, A. Lozano, A. Fasano, S. Kalia, K. Yu, C. Brennan, W. Stemple, N. Abid, M. Yountz, A. Enayetallah, A. Lampron, V. Tabar. Motor and Non-Motor Outcomes of Bemdaneprocel in People With Parkinson’s Disease: Results up to 24 Months From a Phase 1 Study [abstract]. Mov Disord. 2024; 39 (suppl 1). https://www.mdsabstracts.org/abstract/motor-and-non-motor-outcomes-of-bemdaneprocel-in-people-with-parkinsons-disease-results-up-to-24-months-from-a-phase-1-study/. Accessed July 5, 2025.« Back to 2024 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/motor-and-non-motor-outcomes-of-bemdaneprocel-in-people-with-parkinsons-disease-results-up-to-24-months-from-a-phase-1-study/