Objective: This post-hoc analysis of Study CTH-301 assessed the occurrence of oropharyngeal treatment-emergent adverse events (TEAEs) in patients with Parkinson’s disease included in this study.
Background: Study CTH-301 demonstrated that apomorphine sublingual film (SL-APO) was well tolerated and efficacious for treating OFF-episodes in patients with Parkinson’s disease with motor fluctuations.
Method: Study CTH-301 was a Phase 3, multicentre, open-label trial composed of a dose-optimisation (DO) and long-term safety (LTS) phase. Incidence, severity and time to onset of oropharyngeal TEAEs and discontinuations due to oropharyngeal TEAEs were evaluated. Baseline characteristics that differed between patients who developed oropharyngeal TEAEs and those who did not and between patients who discontinued due to oropharyngeal TEAEs and those who did not were also identified.
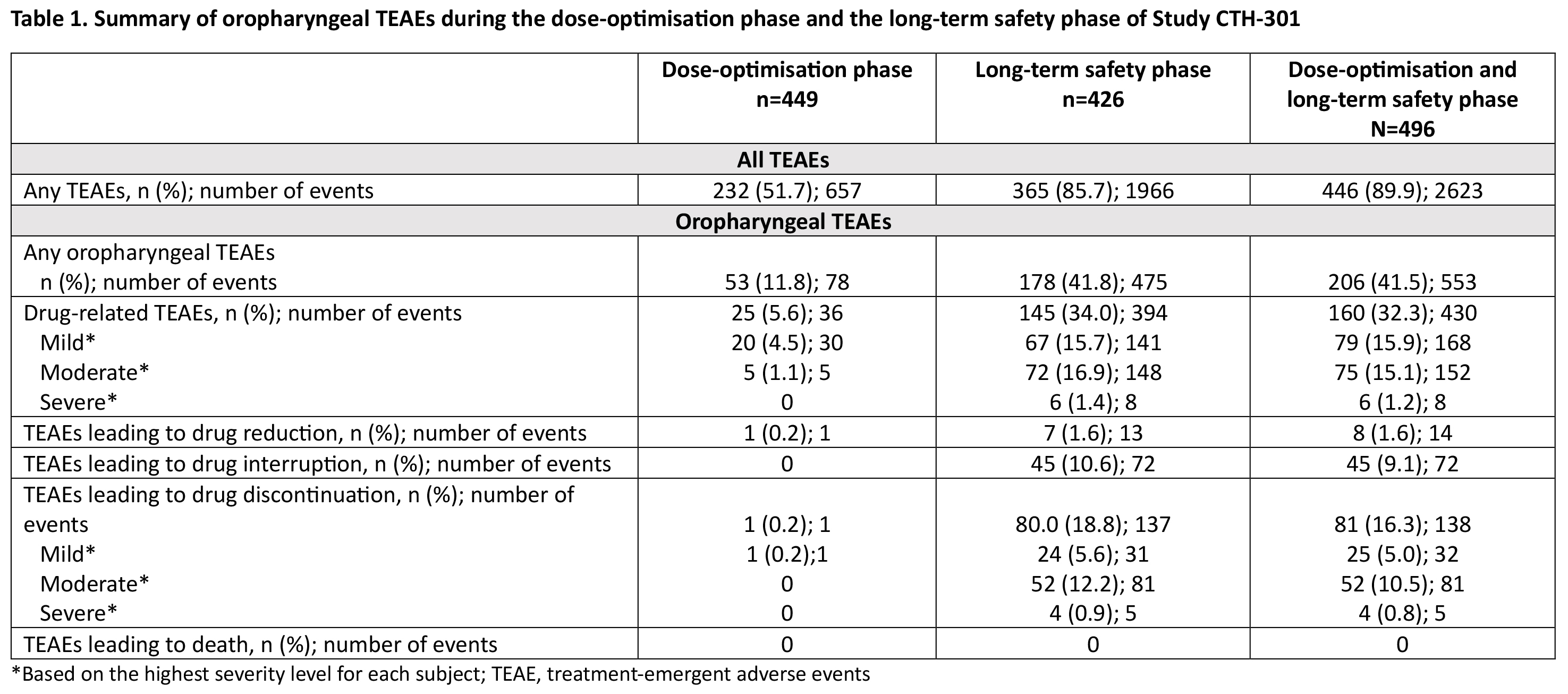
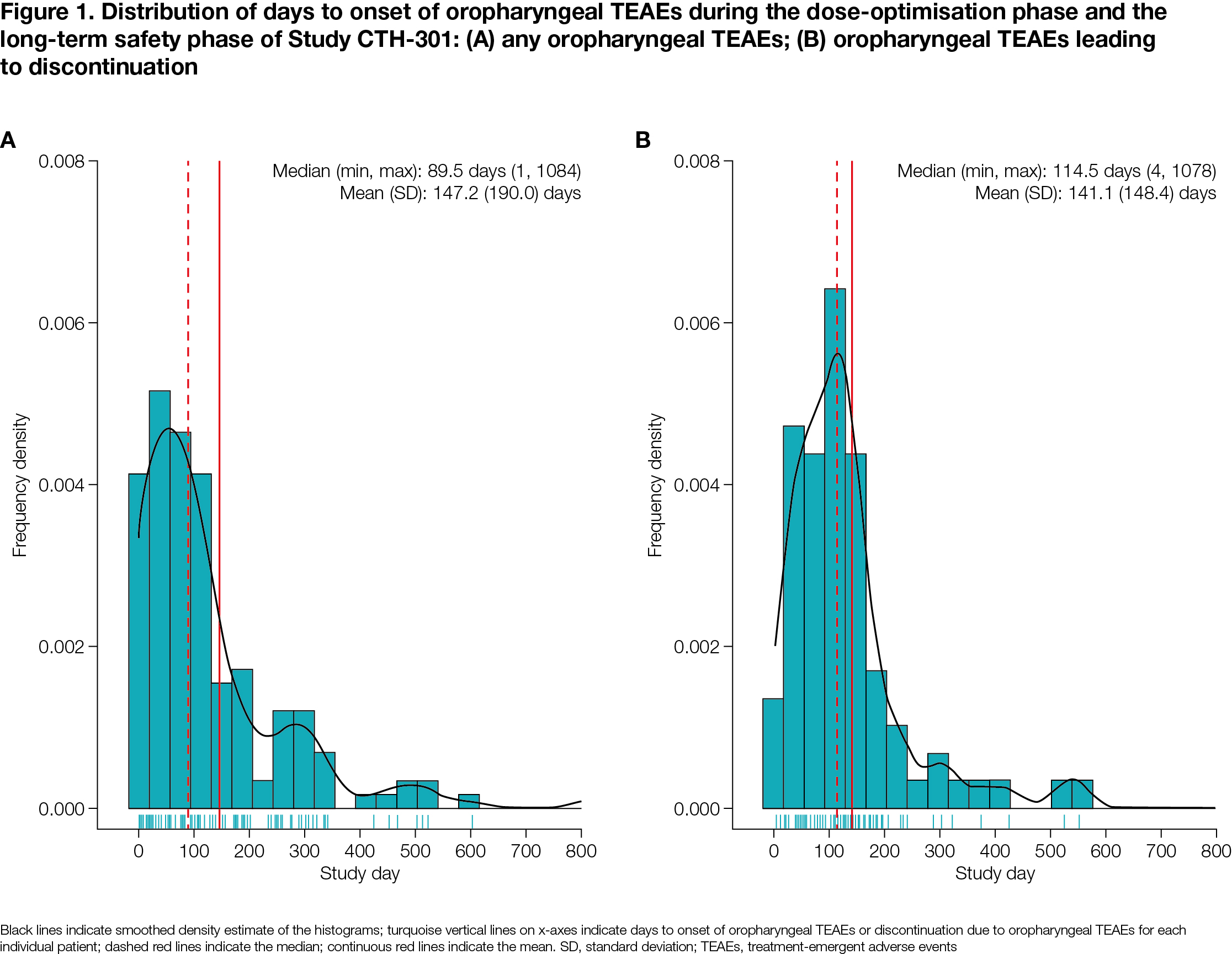
Results: Of 496 patients, 206 (41.5%) experienced oropharyngeal TEAEs and 81 (16.3%) discontinued due to oropharyngeal TEAEs (both DO+LTS) [Table 1]. The majority of oropharyngeal TEAEs overall and those leading to discontinuation were mild or moderate [Table 1], with more severe TEAEs being associated with higher discontinuation rates. Median time to onset for oropharyngeal TEAEs and oropharyngeal TEAEs leading to discontinuation was 89.5 and 114.5 days from study initiation, respectively [Figure 1]. Baseline characteristics significantly associated with the occurrence of oropharyngeal TEAEs were dopamine agonists use (p<0.001), older age (p<0.01) and other dopaminergic medications use (p<0.01). Baseline characteristics significantly associated with occurrence of oropharyngeal TEAEs leading to discontinuation were older age (p<0.01) and dopamine agonists use (p=0.042).
Conclusion: Oropharyngeal TEAEs, including those leading to discontinuation, were mostly mild or moderate and predominantly occurred within the first few months of SL-APO initiation.
Table 1
Figure 1
To cite this abstract in AMA style:
L. Wojtecki, F. Moreira, E. Cubo, M. Fonseca, G. Harrison-Jones, C. Denecke Muhr. Oropharyngeal Adverse Events in Parkinson’s Patients with Motor Fluctuations Treated with Apomorphine Sublingual Film [abstract]. Mov Disord. 2024; 39 (suppl 1). https://www.mdsabstracts.org/abstract/oropharyngeal-adverse-events-in-parkinsons-patients-with-motor-fluctuations-treated-with-apomorphine-sublingual-film/. Accessed July 9, 2025.« Back to 2024 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/oropharyngeal-adverse-events-in-parkinsons-patients-with-motor-fluctuations-treated-with-apomorphine-sublingual-film/