Category: Spasticity
Objective: To assess measures of pain and functional impairment in patients treated with onabotulinumtoxinA (onabotA) who are naive to botulinum toxins with upper limb (UL) and lower limb (LL) spasticity from the Adult Spasticity International Registry (ASPIRE) study.
Background: OnabotulinumtoxinA is indicated for the treatment of spasticity, but limited real-world evidence is available to inform treatment decisions, especially in patients with no prior treatment with botulinum toxins for spasticity.
Method: ASPIRE was a 2-year, international, multicenter, prospective, observational registry study (NCT01930786). Adults with spasticity across multiple etiologies were treated with onabotA during routine clinical practice. Treatments were given at the clinician’s discretion for 8 total treatment sessions over a 96-week period. For this analysis, patients with spastic hemiparesis were defined as receiving ≥1 UL and ≥1 LL treatment with onabotA during the study. Patients who were naive to botulinum toxins for spasticity and who received treatment to both UL and LL at the same treatment session for ≥1 session during the 2-year study are described. Functional impairment and pain were assessed via the Disability Assessment Scale (DAS) and Numeric Pain Rating Scale (NPRS), respectively.
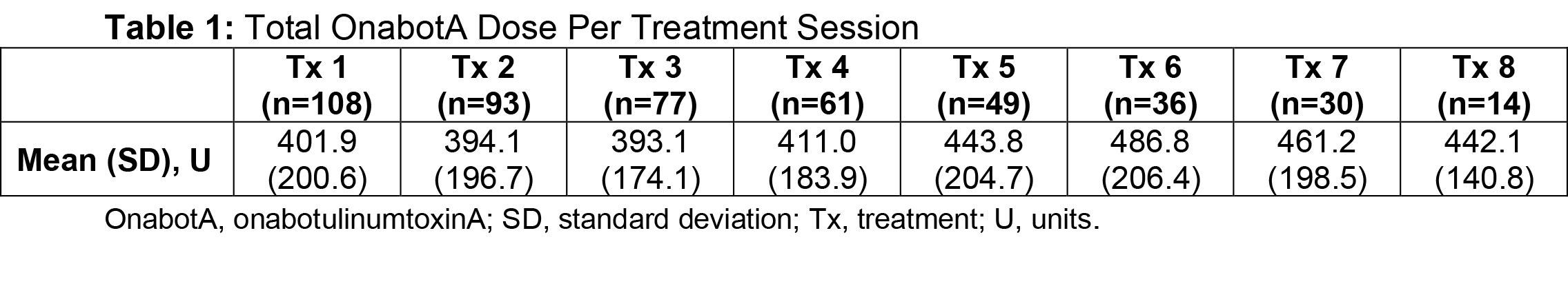
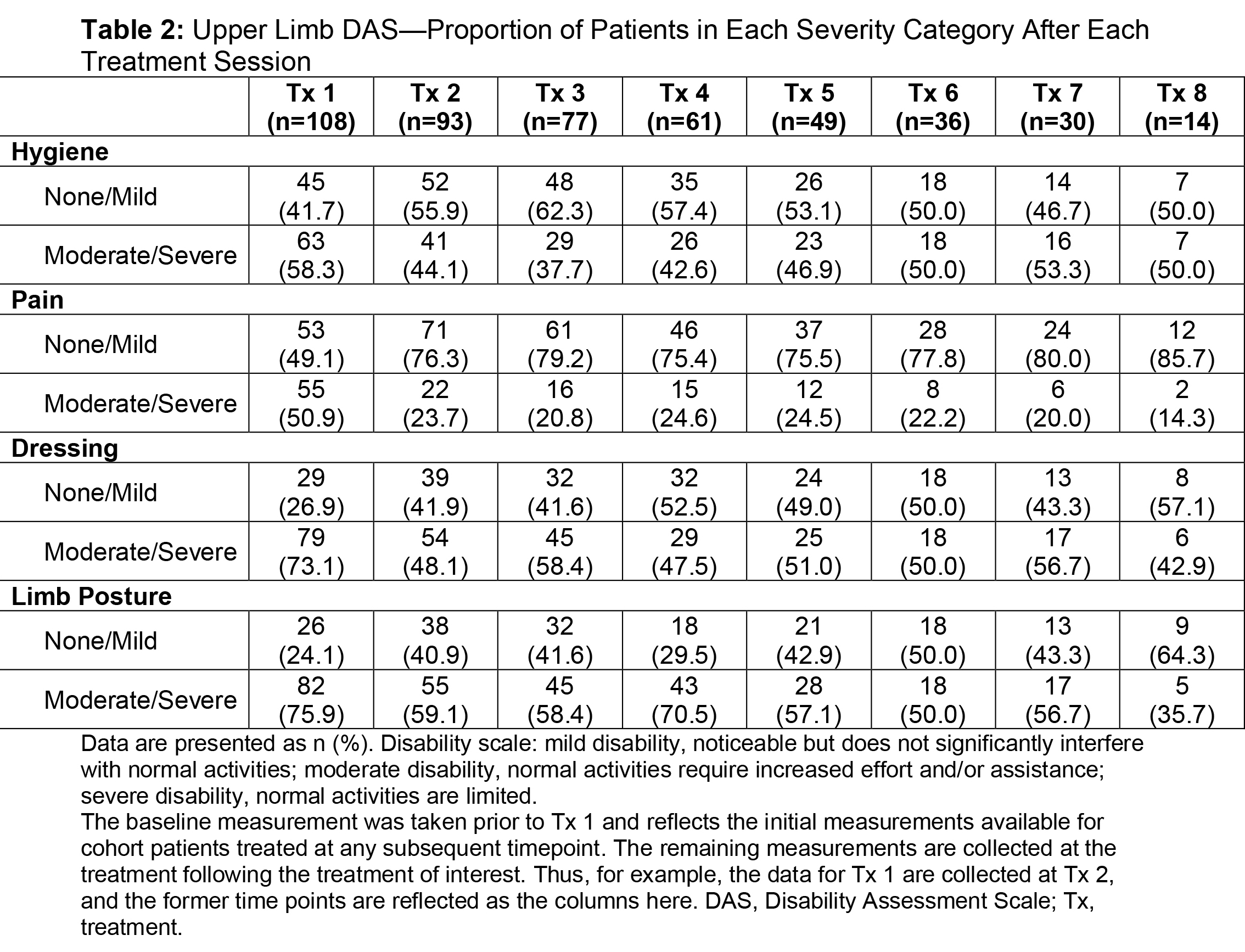
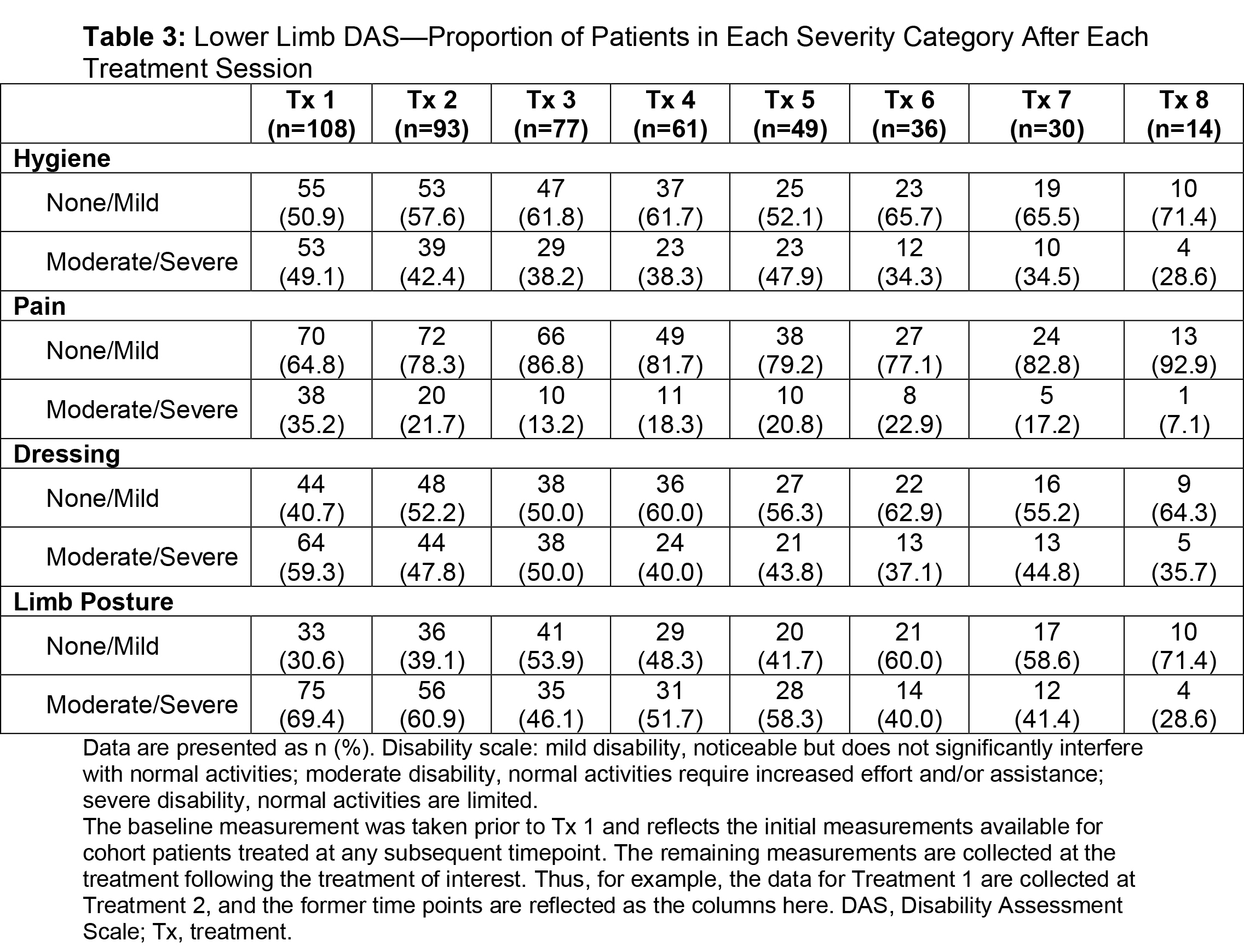
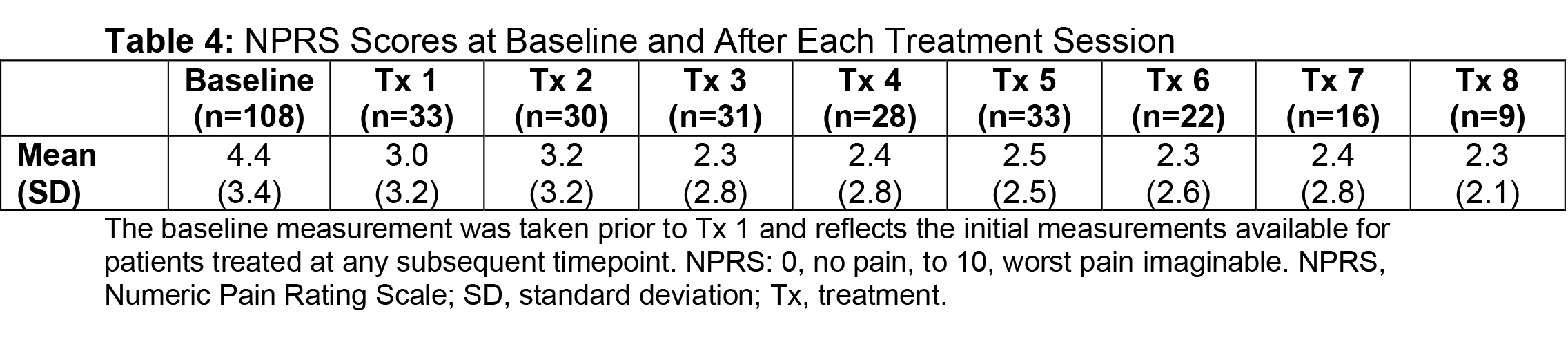
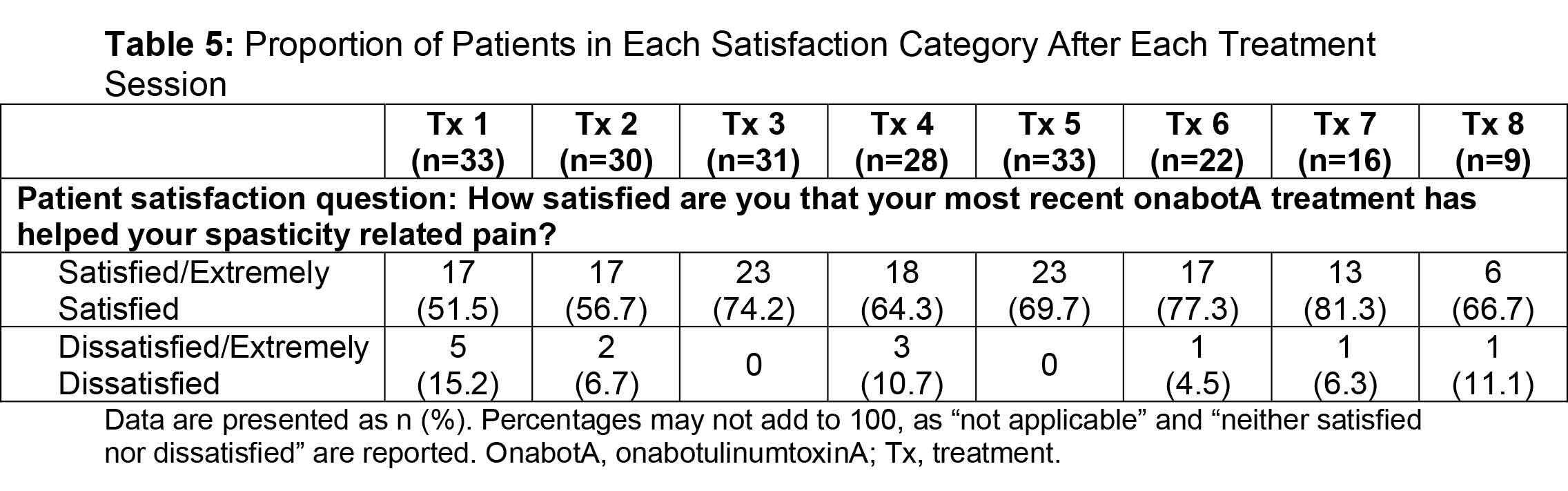
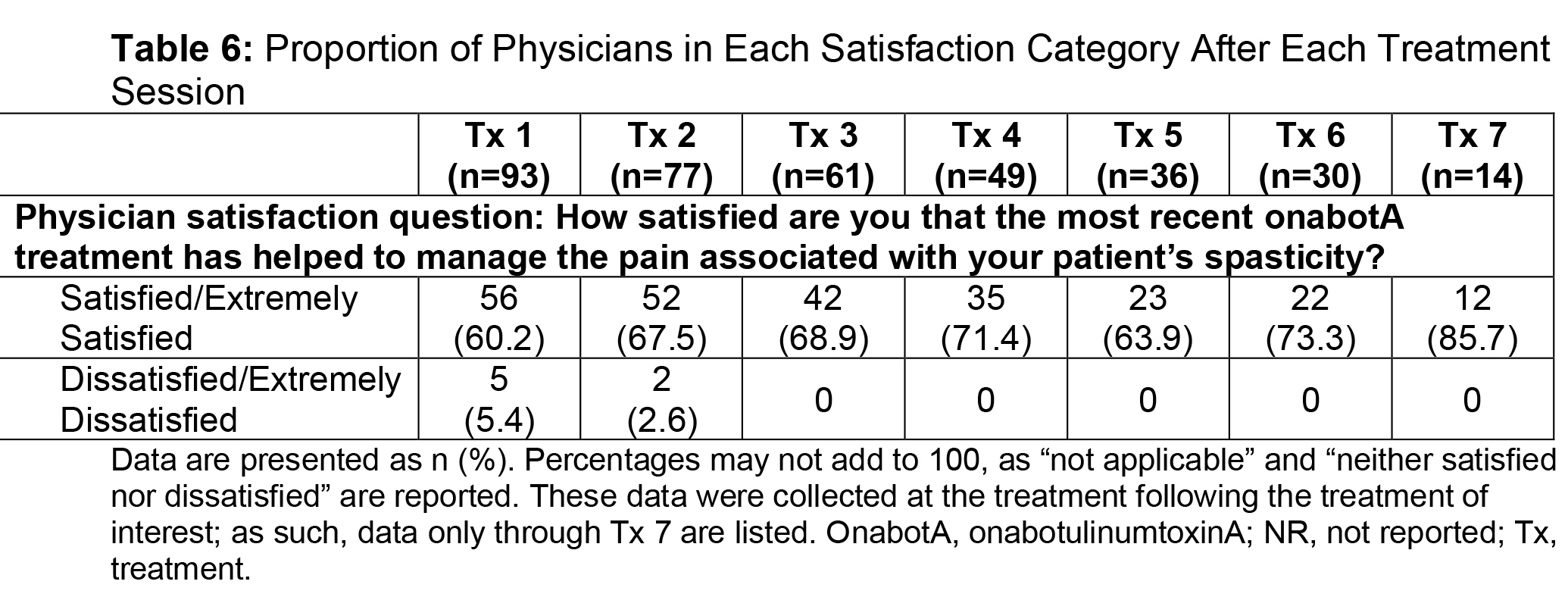
Results: Of the ASPIRE patient population (N=730), 275 hemiparetic patients received treatment for both UL and LL at the same session and 108 of these patients (39.3%) were naive to botulinum toxins for spasticity. For this subgroup, average age was 55.2 years (range, 22.5-88.5), 51.9% were male, 62.0% Caucasian, and 75.9% post-stroke. The total mean onabotA dose per treatment session ranged from 393.1 to 486.8 U [table1]. Throughout the treatment sessions, patients demonstrated gradual improvement in functional impairment based on the DAS in both UL and LL [table2, table3]. The mean NPRS score was reduced from baseline at each treatment session [table4]. Most patients and physicians were satisfied/extremely satisfied with onabotA treatment throughout the sessions based on questionnaire reports [table5, table6].
Conclusion: In this subset analysis of ASPIRE patients who are naive to botulinum toxins for spasticity, onabotA treatment reduced functional impairment and pain, as assessed by the DAS and NPRS, respectively.
To cite this abstract in AMA style:
G. Bavikatte, A. Esquenazi, W. Jost, A. Barichich, E. Duarte, M. Schwartz, T. Musacchio, G. Francisco. Pain and Disability Outcomes in Hemiparetic Patients Naive to Botulinum Toxins With Upper and Lower Limb Spasticity Treated With OnabotulinumtoxinA: Insights From the ASPIRE Study [abstract]. Mov Disord. 2022; 37 (suppl 2). https://www.mdsabstracts.org/abstract/pain-and-disability-outcomes-in-hemiparetic-patients-naive-to-botulinum-toxins-with-upper-and-lower-limb-spasticity-treated-with-onabotulinumtoxina-insights-from-the-aspire-study/. Accessed July 18, 2025.« Back to 2022 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/pain-and-disability-outcomes-in-hemiparetic-patients-naive-to-botulinum-toxins-with-upper-and-lower-limb-spasticity-treated-with-onabotulinumtoxina-insights-from-the-aspire-study/