Session Information
Date: Sunday, October 7, 2018
Session Title: Huntington's Disease
Session Time: 1:45pm-3:15pm
Location: Hall 3FG
Objective: To assess cerebrospinal fluid (CSF) mutant Huntingtin (mHTT), and CSF and plasma neurofilament light (NfL) in Huntington’s disease (HD).
Background: HD is a progressive neurodegenerative disorder where there is a pressing need for sensitive biomarkers. These two proteins – mHTT as the pathogenic agent and a pharmacodynamic marker of huntingtin-lowering, and NfL as a marker of neuronal damage – have the potential to form a powerful biofluid biomarker combination to facilitate disease-modifying trials in HD. Here we assess them in parallel for the first time, and against clinical and neuroimaging outcome measures.
Methods: Eighty participants (20 healthy controls, 20 premanifest HD and 40 manifest HD) were recruited. All underwent clinical assessments, and standardized CSF and blood collections. Some had an optional brain MRI and a second sample collection. CSF mHTT, CSF NfL and plasma NfL were measured using immunoassays. T1-weighted MRI data were acquired. We computed age- and age-and-CAG-adjusted intergroup comparisons and correlations, using multiple linear regression models and Pearson’s correlations. Receiver operating characteristics curves and samples sizes were also derived to study the analytes’ properties.
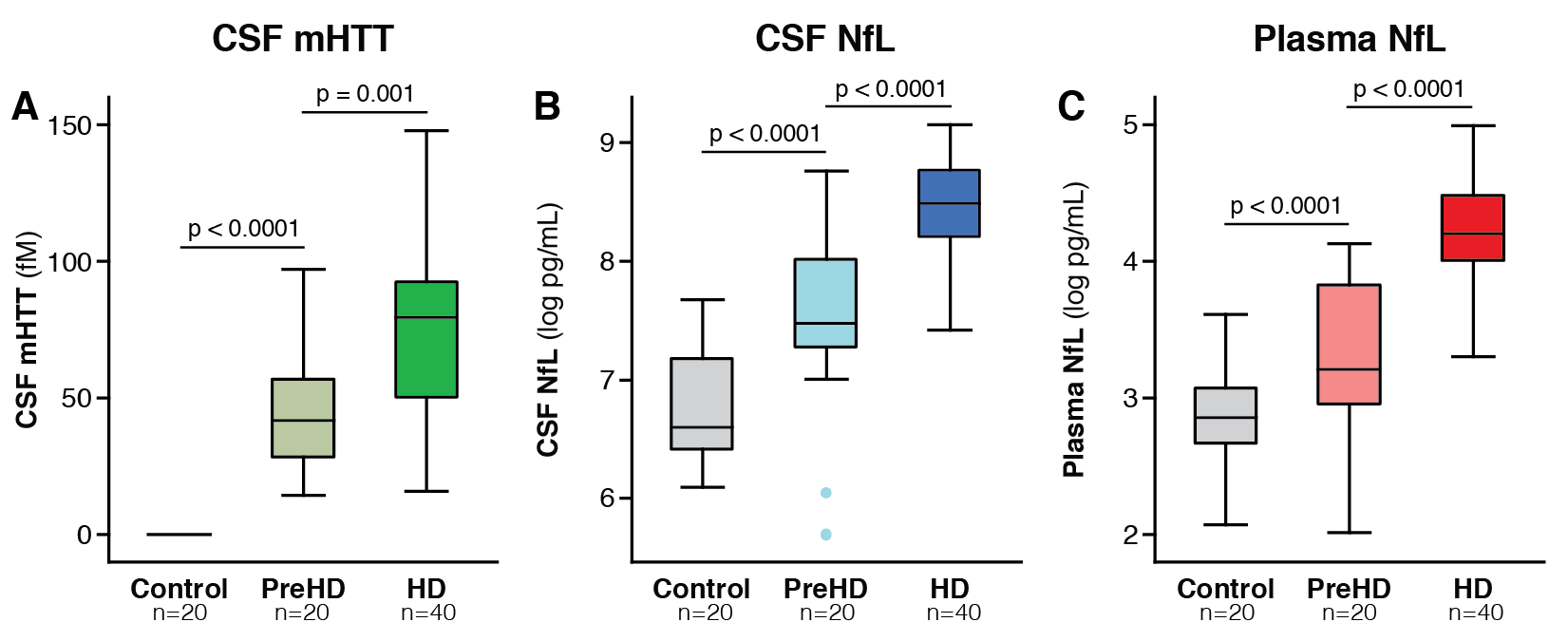
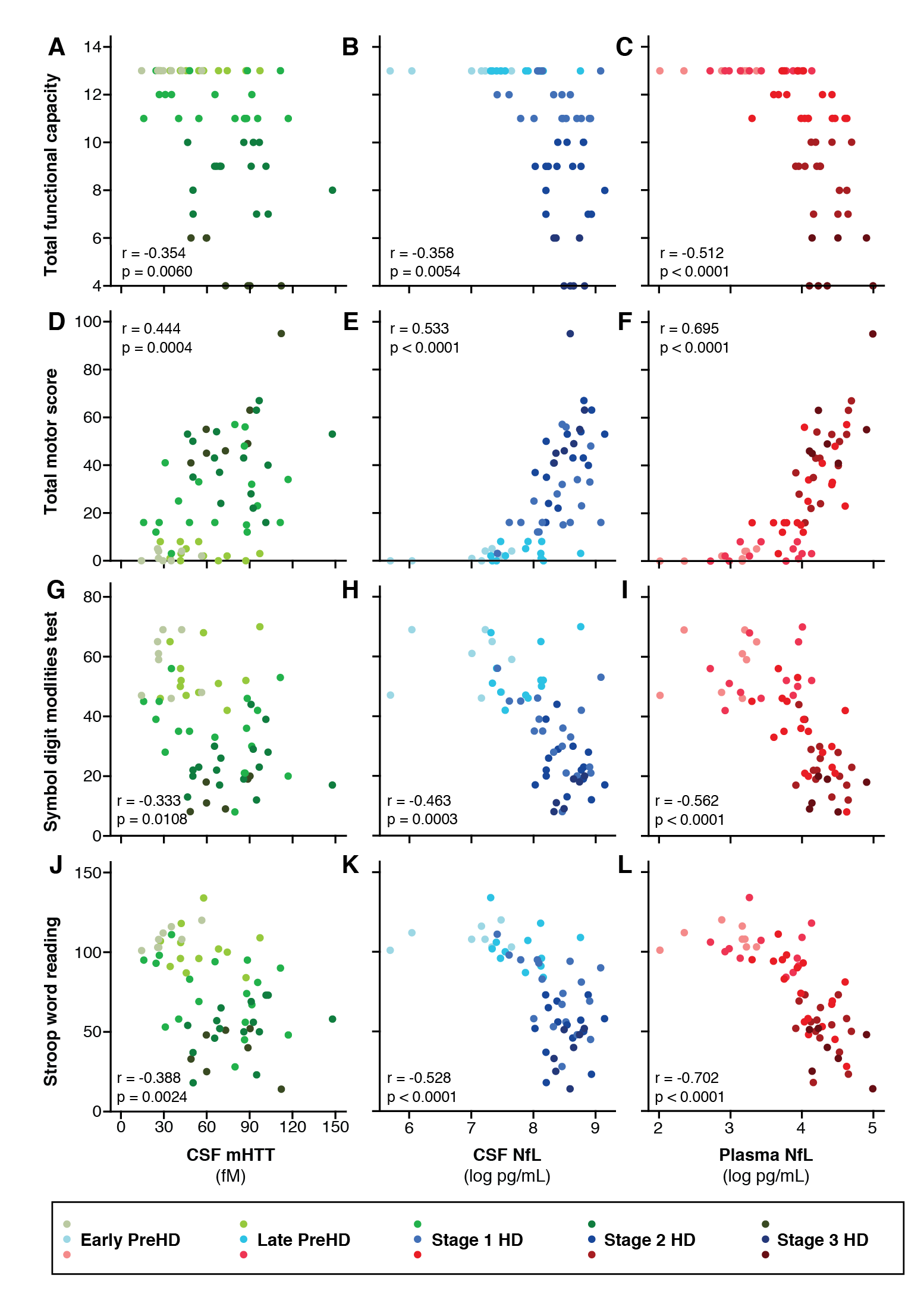
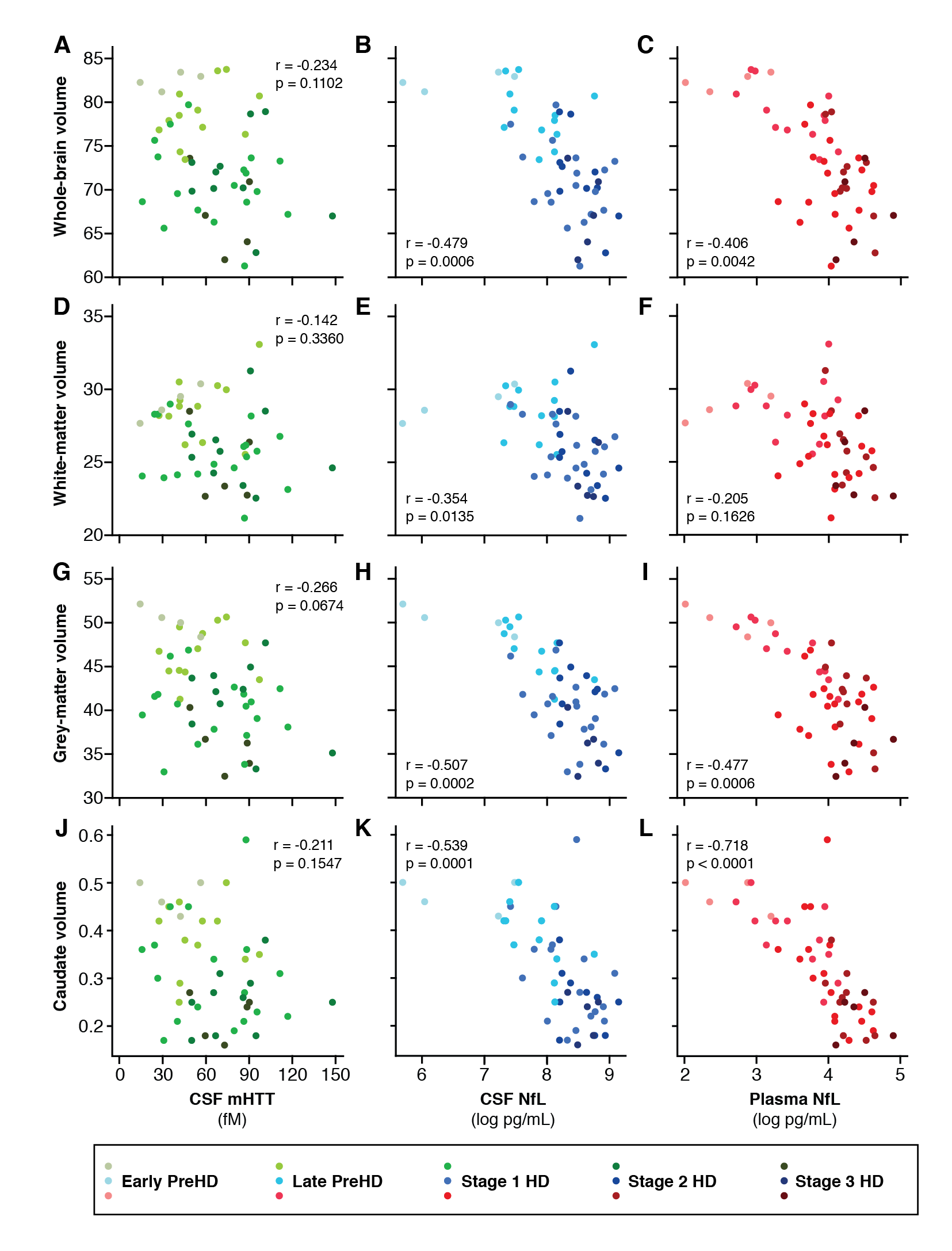
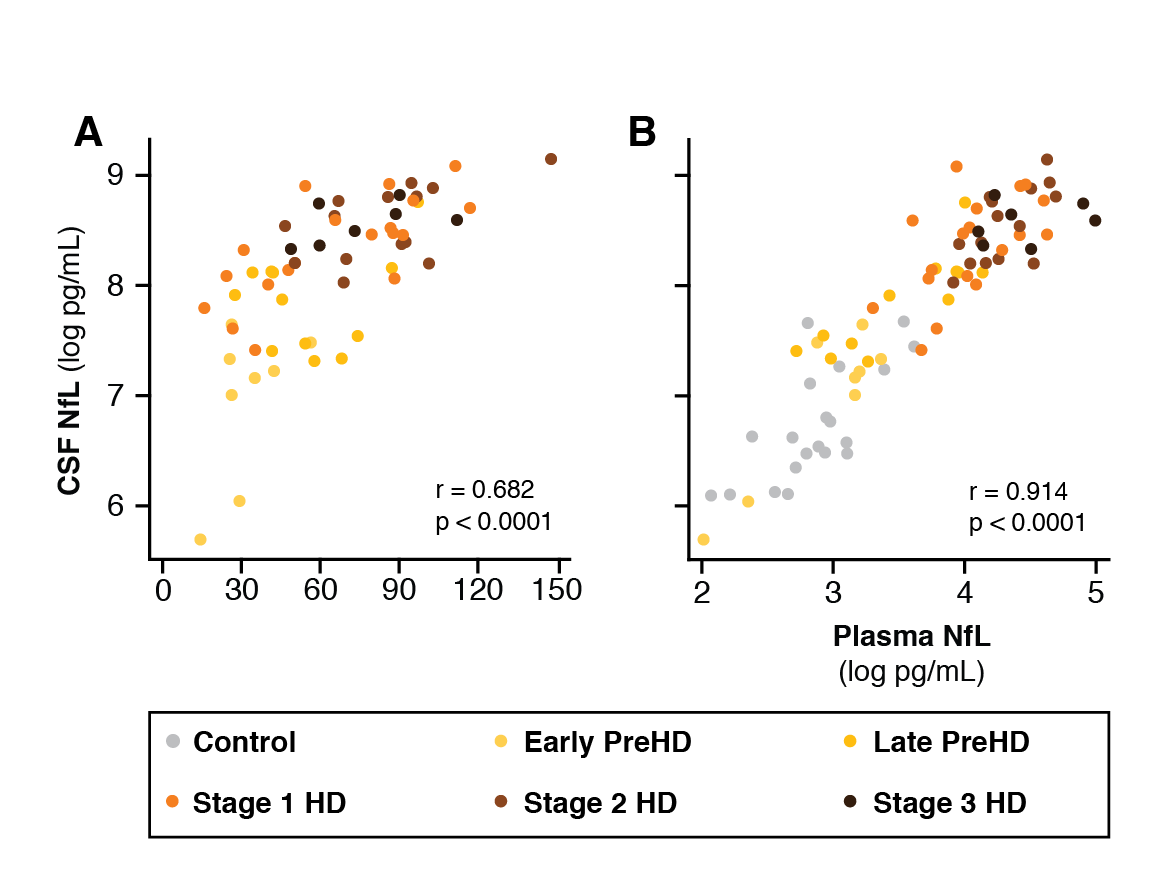
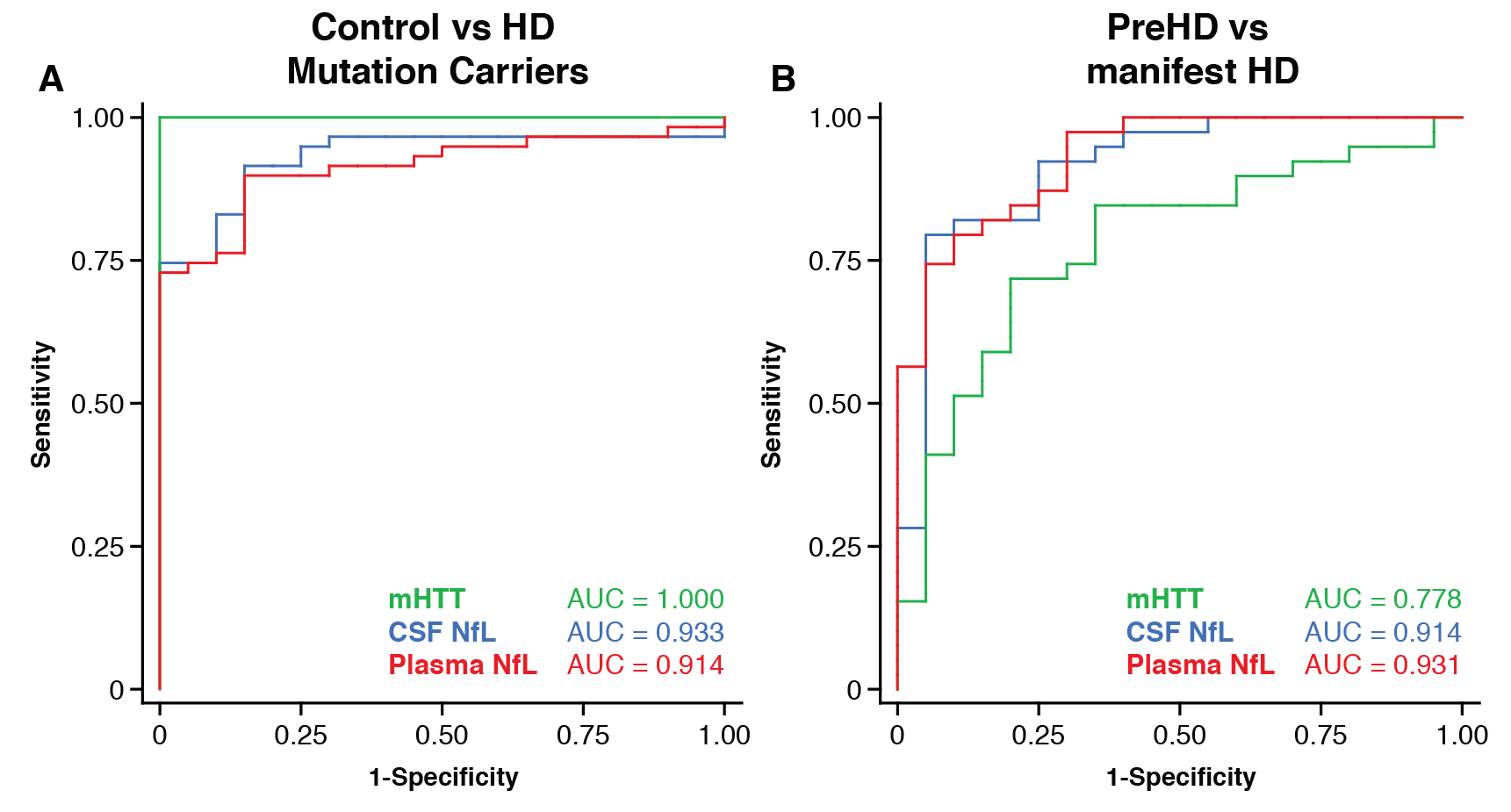
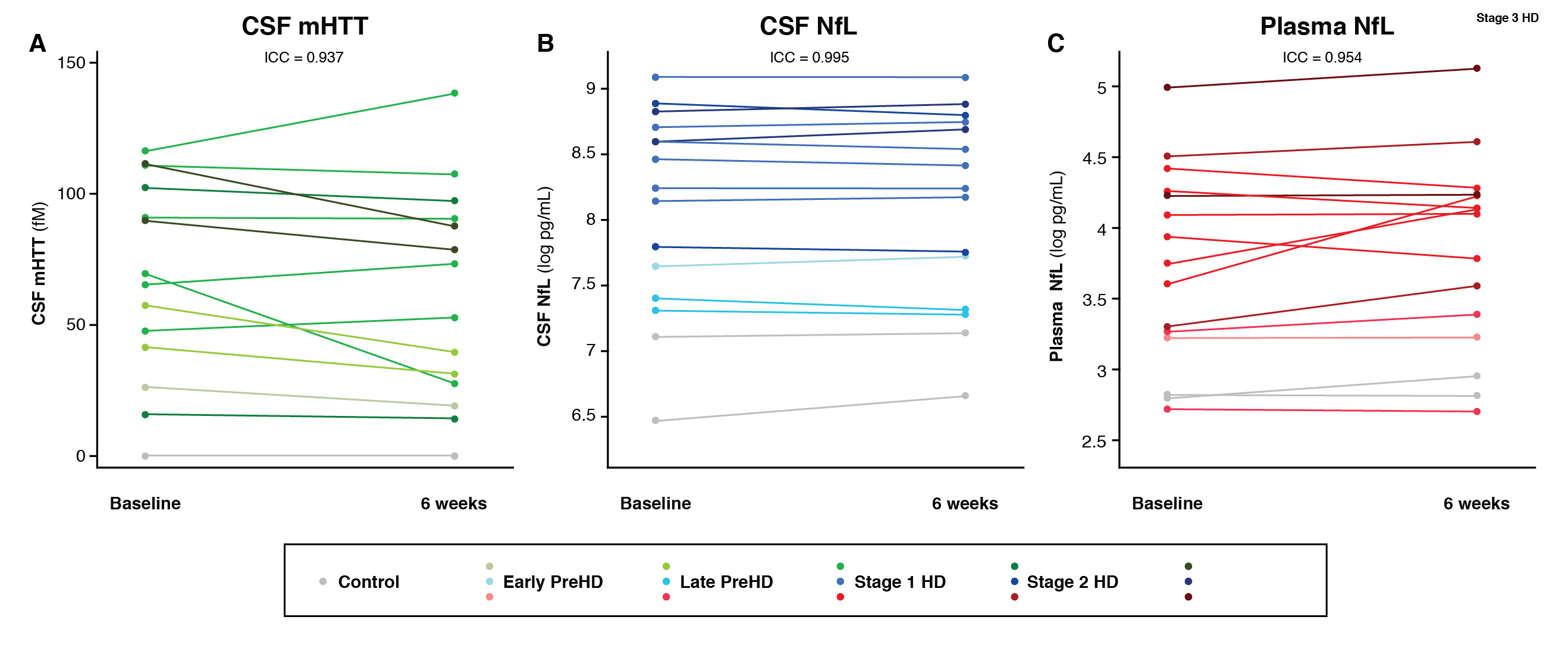
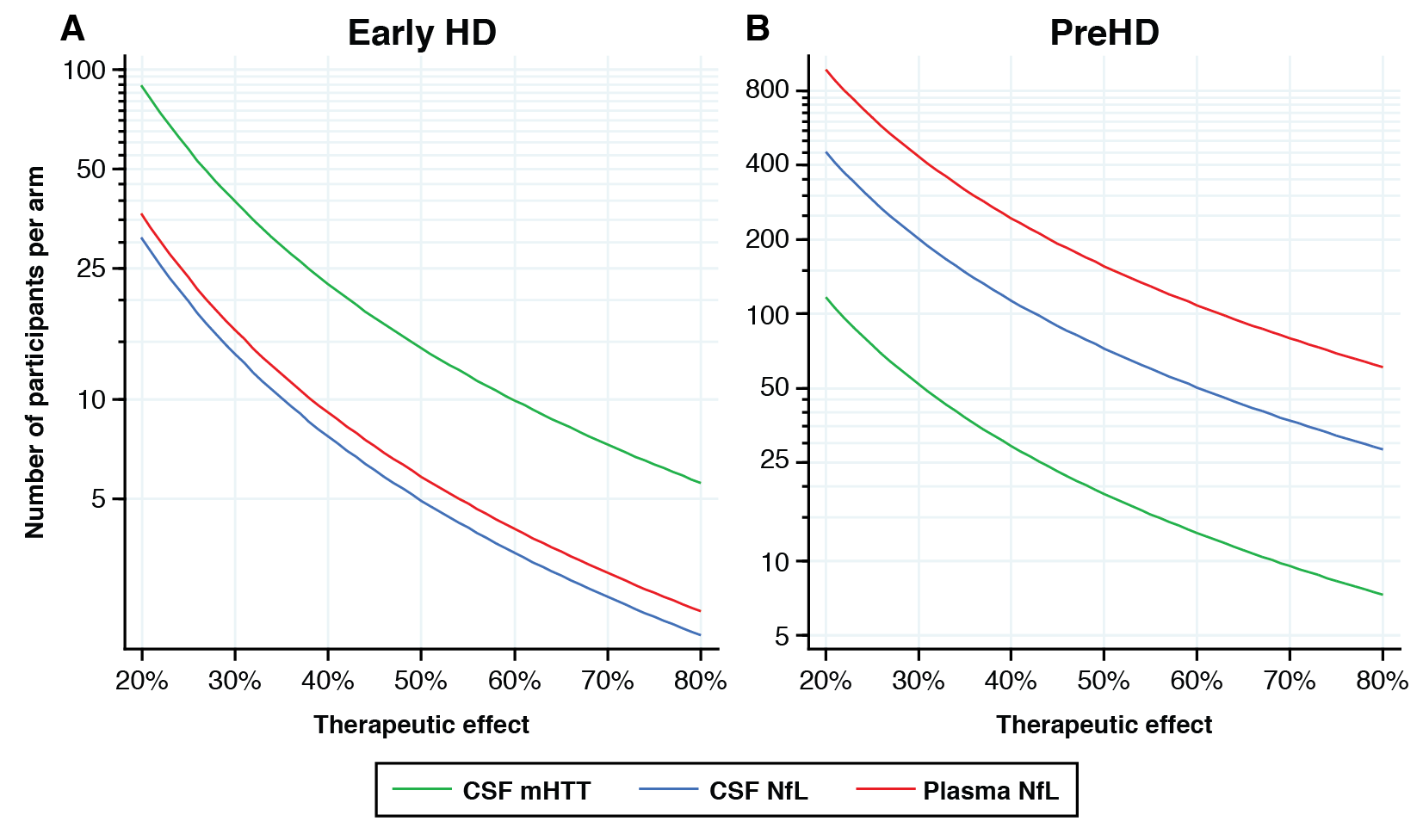
Results: CSF mHTT, CSF NfL and plasma NfL were all significantly higher in HD mutation carriers compared to controls, and significantly rose with disease stage. Among HD mutation carriers, there were significant associations with all clinical measures assessed. CSF NfL was associated with all MRI measures, while plasma NfL was associated with whole brain and grey matter volume. CSF mHTT was not associated with brain volume. CSF mHTT, CSF NfL and plasma NfL were closely correlated, and highly stable within individuals. CSF mHTT had perfect accuracy for distinguishing between controls and HD mutation carriers, and NfL, both in CSF and plasma, had excellent accuracy for distinguishing between premanifest and manifest HD. Sample size calculations suggested these measures can be incorporated into clinical trials without the need to recruit additional participants.
Conclusions: In this cross-sectional study we provide evidence to support two proteins, mHTT and NfL, as having favourable properties as biofluid biomarkers for HD.
To cite this abstract in AMA style:
L. Byrne, F. Rodrigues, E. Johnson, E. De Vita, C. Czech, S. Schobel, R. Scahill, A. Heslegrave, H. Zetterberg, W. Wild. Parallel evaluation of mutant huntingtin and neurofilament light in Huntington’s disease [abstract]. Mov Disord. 2018; 33 (suppl 2). https://www.mdsabstracts.org/abstract/parallel-evaluation-of-mutant-huntingtin-and-neurofilament-light-in-huntingtons-disease/. Accessed July 9, 2025.« Back to 2018 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/parallel-evaluation-of-mutant-huntingtin-and-neurofilament-light-in-huntingtons-disease/