Objective: To describe the study design and timeline of the first interventional study in Neuronal α-Synuclein Disease (NSD).
Background: P2P is a platform, Phase 2 randomized double blind multi-center, multi-regimen clinical trial that is planned to evaluate the safety and early efficacy of investigational products in participants with NSD stage 2B.
NSD is defined by presence of Alpha-Synuclein pathology, ultimately dopamine dysfunction, and stage dependent motor and non-motor clinical manifestations and related functional impairment. These participants were previously clinically defined as Parkinson’s disease, Dementia with Lewy Bodies and Prodromal. The study is “nested” within the PPMI and sponsored by the MJFF.
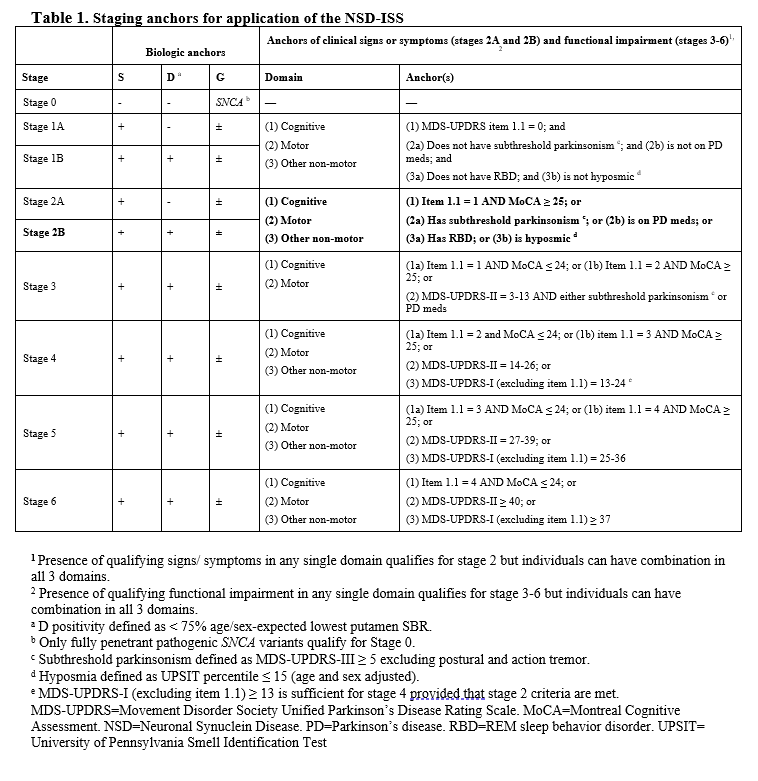
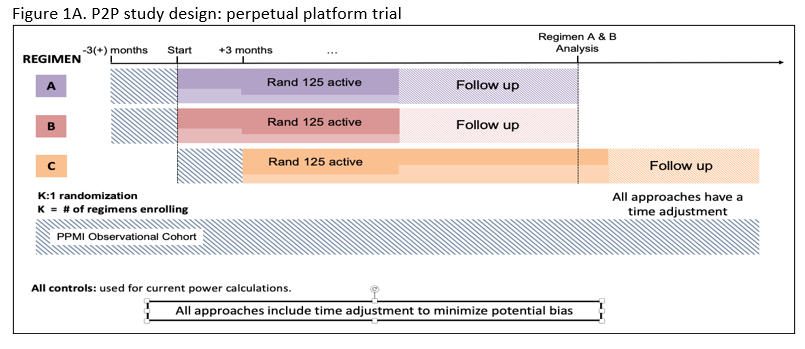
Method: P2P is a perpetual platform trial with a single Master Protocol dictating the conduct of the trial and regimen specific subprotocols outlining intervention specific aspects for each arm (Figure 1A). Qualified participants will be recruited from the PPMI participants, based on NSD Stage 2B criteria ( Table 1).
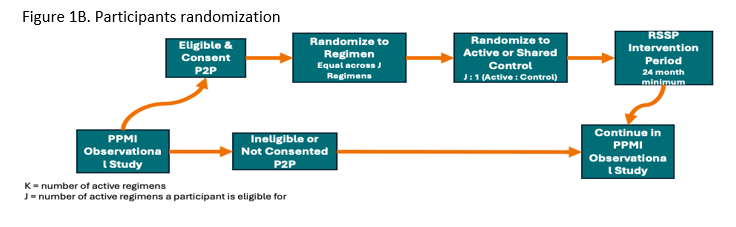
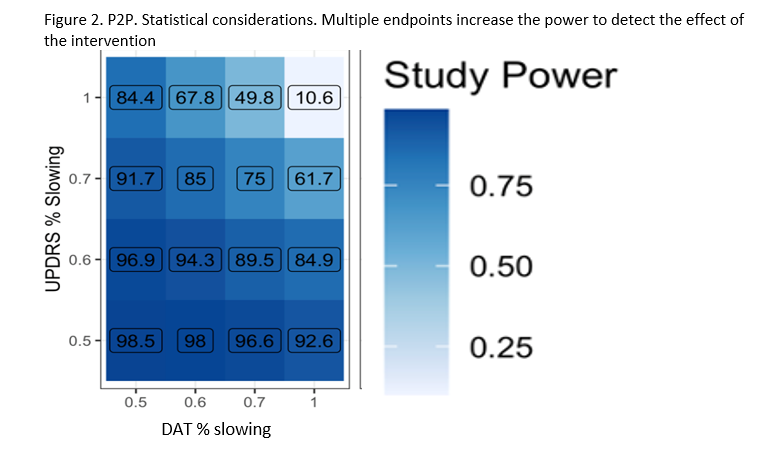
The study’s Multiple Primary Endpoints include 1) DAT imaging as measured by the rate of progression in the mean striatum Specific Binding Ratio (SBR) and 2) rate of progression in the MDS-UPDRS part III score. Secondary endpoints include safety, tolerability and feasibility. The study will have an array of exploratory clinical (including digital) and biomarker measures. Participants will first be randomized in an equal manner among all of the regimen-specific sub-protocols for which they are eligible. After randomization to a specific subprotocol, participants will be randomized to an active arm or placebo (N=125 per arm) in a K:1 ratio with K denoting the number of active interventions. Intervention duration will be at least 24 months (until the last participant in that regimen completes 24 months). The study is 85% powered to detect a slowing in either primary endpoint for each regimen, assuming a 40 % slowing in either endpoint (Figure 2).
Results: Final selection of the first 2 interventions will be presented from > 15 industry submitted applications. The study targets to start enrolment in the first 2 regimens in 2025[TS1] .
[TS1]We could add PPMI has recruited N=XX of potetially qualified participants
Conclusion: We report the design of the first platform interventional study targeting NSD Stage 2B population. Innovative recruitment into PPMI will enable this study.
Table 1
Figure 1A
Figure 1B
Figure 2
To cite this abstract in AMA style:
T. Simuni, C. Coffey, A. Siderowf, C. Tanner, S. Chowdhury, T. Sherer, C. Kopil, K. Kieburtz, B. Saville, C. Allen-Savietta, B. Wendelberger, A. Crawford, K. Fabrizio, K. Marek. Path To Prevention (P2P) Therapeutics Platform Trial in Stage 2B Neuronal Alpha-Synuclein Disease: Study Update [abstract]. Mov Disord. 2024; 39 (suppl 1). https://www.mdsabstracts.org/abstract/path-to-prevention-p2p-therapeutics-platform-trial-in-stage-2b-neuronal-alpha-synuclein-disease-study-update/. Accessed July 13, 2025.« Back to 2024 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/path-to-prevention-p2p-therapeutics-platform-trial-in-stage-2b-neuronal-alpha-synuclein-disease-study-update/