Category: Parkinson’s Disease: Clinical Trials
Objective: The overall aim is to compare the efficacy of using wearable devices versus standard of care in improving the timeliness of referrals to evaluation for advanced treatments for people with Parkinsons Disease (PD).
Background: Due to an aging global population, the prevalence of PD is on the rise, presenting a diverse range of fluctuating symptoms and unique challenges to affected individuals. One of the key issues in PD management is infrequent clinical visit combined with the heavy reliance on subjective symptom reports from patients or their caregivers. The fluctuating nature of PD, compounded by biases like recall and report bias, challenges the accurate assessment and monitoring of PD symptoms in the patients’ daily life. Currently, there exist no clear objective measures for disease outcomes and even clinical motor scales show significant inter-rater variability.
To address these challenges, digital biomarkers and wearable devices have shown potential to support PD care and referral to advanced PD therapy. These devices are generally well accepted by patients and can provide continuous and objective monitoring of PD motor symptoms, allowing for personalized treatment planning and reducing biases associated with subjective reporting.
Method: The study is a randomized, controlled, multicenter trial. Participants will be recruited from the uptake area of three movement disorder clinics and four private practice neurologists in Denmark, and randomized to one of two arms:
Intervention group: Participants will wear a wrist worn sensor and a mobile app to report symptoms.
Control group: Participants will wear a wrist worn sensor, but they and their healthcare provider will be blinded to the data during the study.
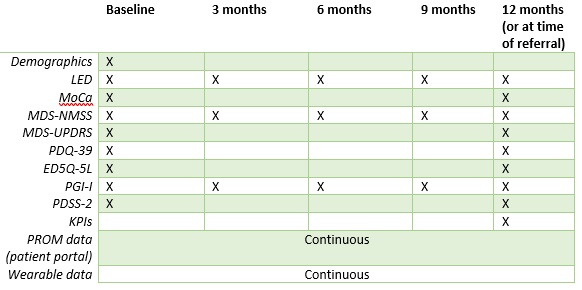
Both groups will have 5 visits during the study period, where data from self-reported questionnaires, clinical test, PROM, and the wearable device will be evaluated.
Results: Results from an initial pilot study and a survey addressing the motivation and barriers for digital devices from neurologists in Denmark will be presented at the MDS Conference 2024.
Conclusion: We expect the study to show earlier referral timings, indicating better identification of patients relevant for advanced treatment.
Table of data collection points
To cite this abstract in AMA style:
N. Karottki, T. Hørmann Thomsen, P. Jennum, M. Blaabjerg, B. Biering-Sørensen. PD-DigiCare: Enhancing referral decision-making for advanced treatment in Parkinson’s Disease through objective measurements and patient reported outcomes – a multicenter randomized controlled trial. [abstract]. Mov Disord. 2024; 39 (suppl 1). https://www.mdsabstracts.org/abstract/pd-digicare-enhancing-referral-decision-making-for-advanced-treatment-in-parkinsons-disease-through-objective-measurements-and-patient-reported-outcomes-a-multicenter-randomized-controlled-trial/. Accessed July 12, 2025.« Back to 2024 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/pd-digicare-enhancing-referral-decision-making-for-advanced-treatment-in-parkinsons-disease-through-objective-measurements-and-patient-reported-outcomes-a-multicenter-randomized-controlled-trial/