Session Information
Date: Monday, October 8, 2018
Session Title: Rating Scales
Session Time: 1:15pm-2:45pm
Location: Hall 3FG
Objective: Our objective was to develop and pilot test a scale (OPTIPARK) for assessing a patient´s dopaminergic state, allowing the clinician to consider the adjustment of treatment to the patient’s needs.
Background: Parkinson´s disease (PD) is a chronic disorder due to a progressive loss of dopaminergic neurons within the substantia nigra pars compacta in the brainstem (1). At early and mid-stages of the disease, medical therapy with dopamine replacement is effective at minimizing most of the motor and non-motor symptoms (2). As neurodegeneration progresses, dopaminergic treatment should be raised and in most patients surgical or infusion therapies are considered at some point (2).
Methods: We designed a preliminary version of the scale that includes nine items (maximal score, 18) encompassing motor (wearing-off, no-on, off-time, dyskinesia, tremor) and non-motor (anxiety, pain, fatigue) complications as well as disability. Each item is scored from 0 to 2 according to patient´s impairment; none (0), mild to moderate (1) or severe (2). Thirty patients answered the preliminary version and a questionnaire about the scale in a unicenter, observational, cross-sectional study. The OPTIPARK feasibility/acceptability and preliminary concordance with the criterion (independent decision to adjust treatment by an expert in movement disorders) were analyzed. For the latter attribute, weighted kappa (with quadratic weights) was applied.
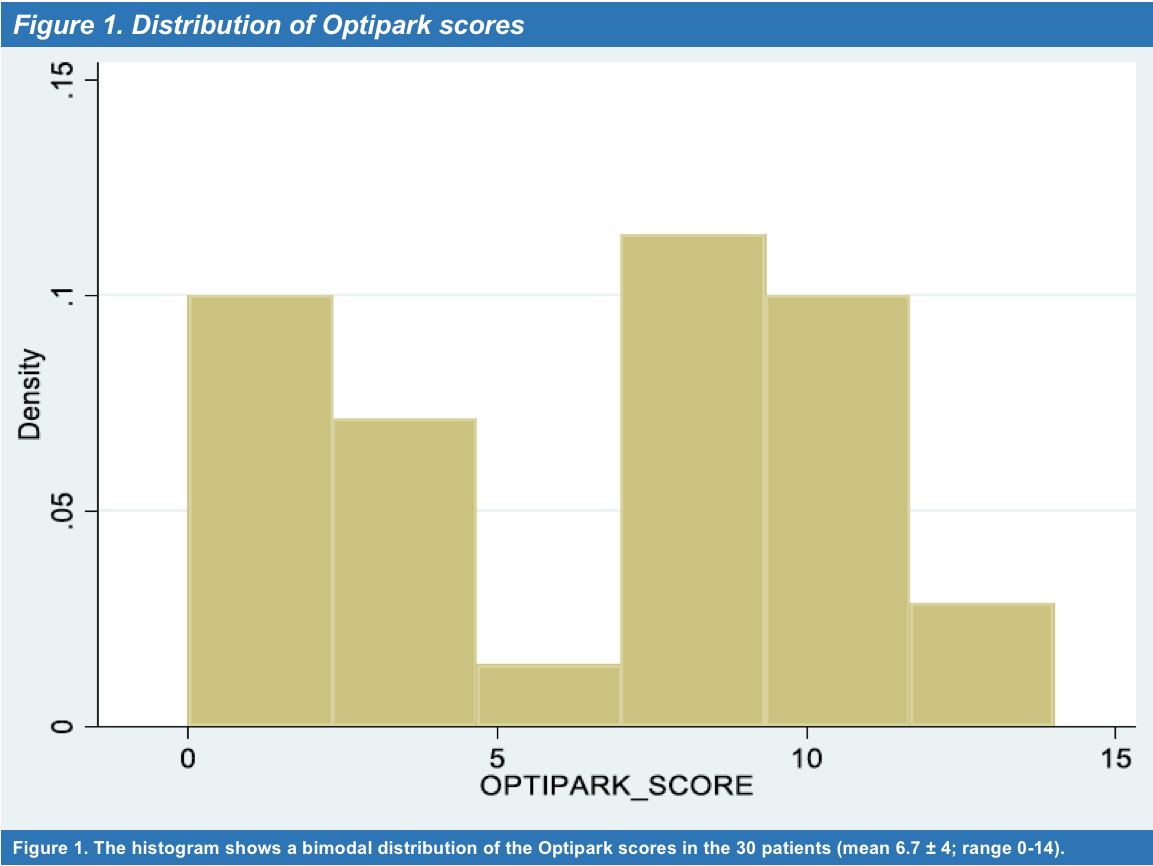
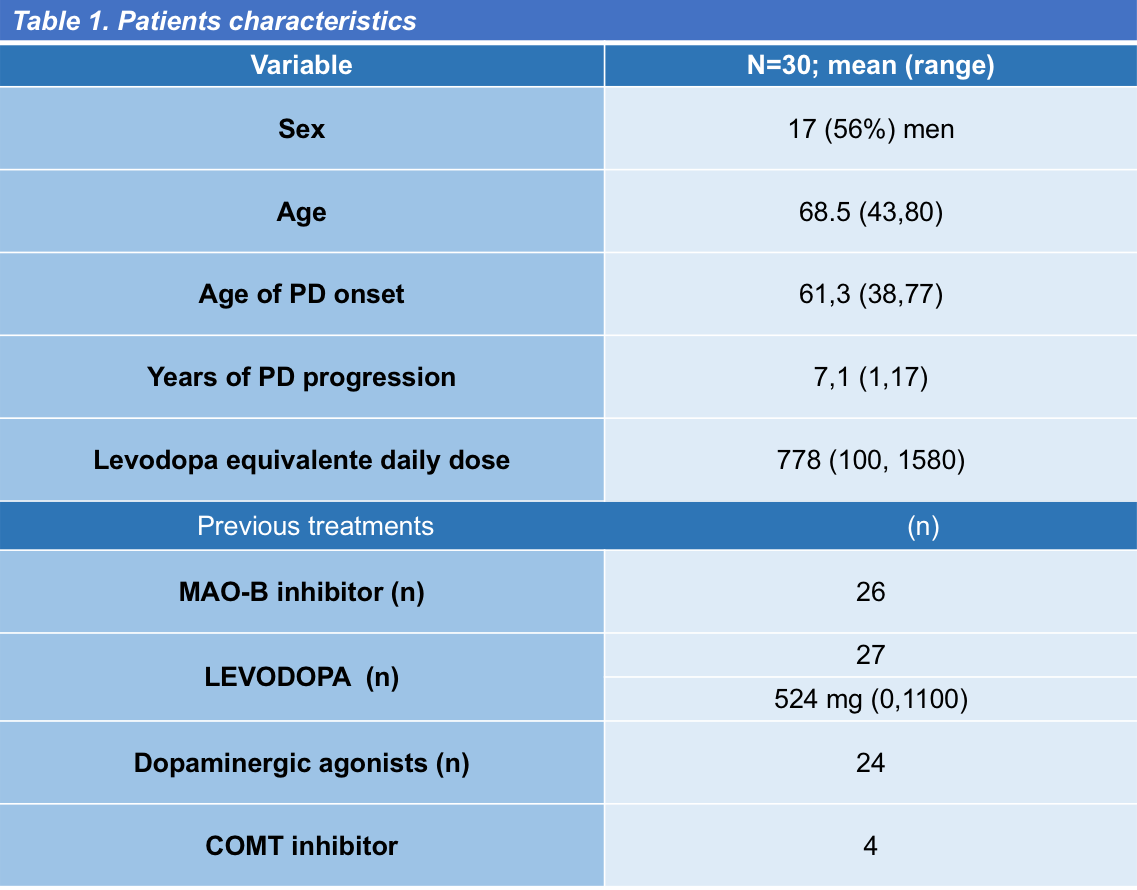
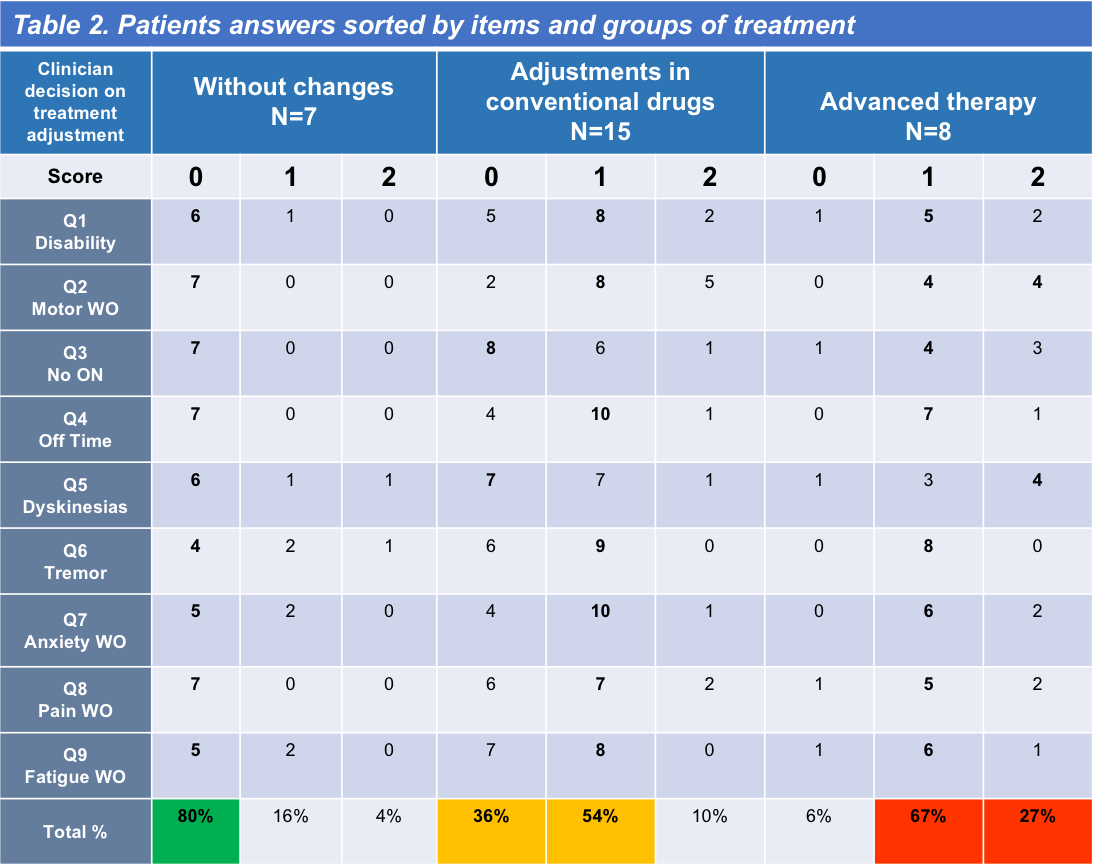
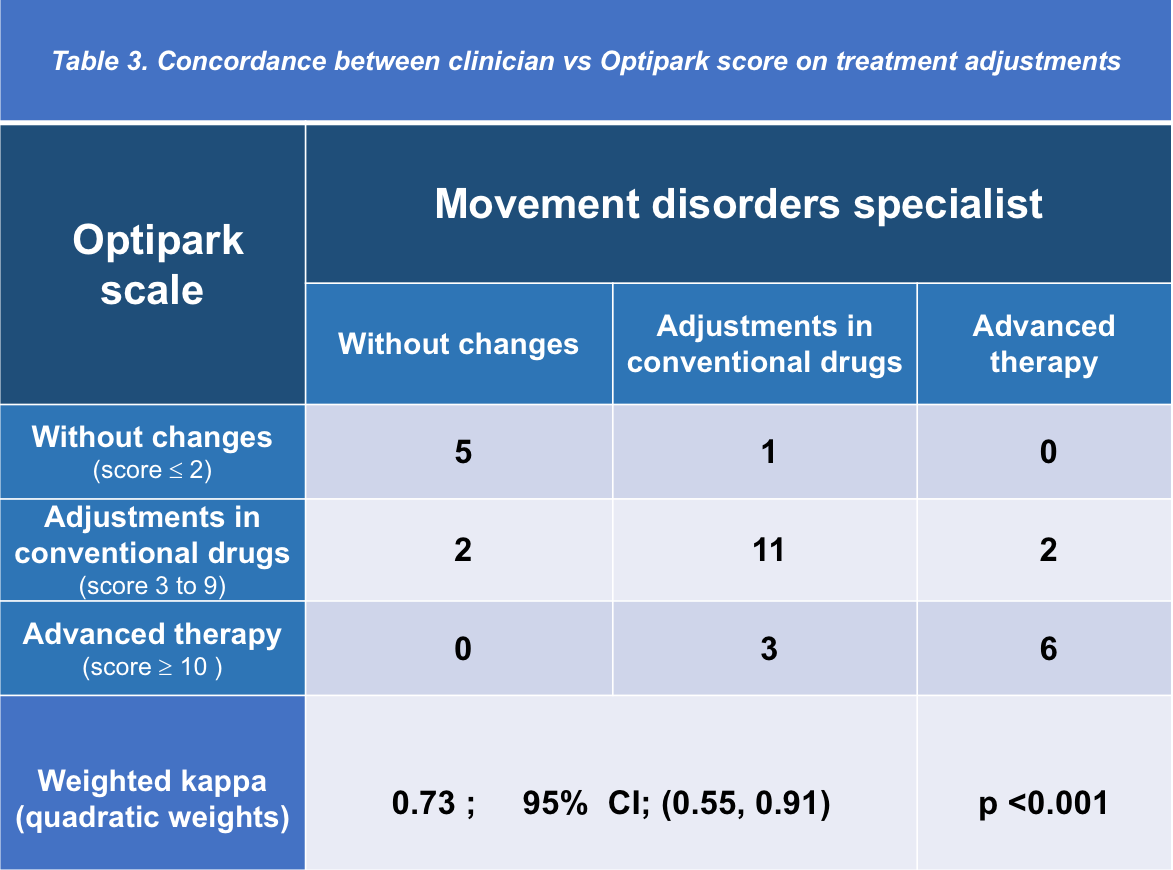
Results: Thirty PD patients (68.5 ± 7.5 years; range, 43-80 years) in Hoehn-Yahr stages I to III completed the scale (mean score 6.7 ± 4; range 0-14) [Figure 1] and the feed-back questionnaire. For patient´s main characteristics see [Table 1]. Only one patient found the scale difficult and too long to answer. Five new items were suggested to be included, none of them by more than one patient. Clinician decisions were classified as “without changes” (mean score 1.5 ± 0.9; range 0-3), “adjustments in conventional drugs” (mean score 7 ± 2.8; range 2-11), and “infusion or surgical therapies” (mean score 10.8 ± 1.8; range 9-14). Patient´s answers sorted per item and clinician decision are shown in [Table 2]. Setting [0,2] for “without changes,” [3-9] “adjustments in conventional drugs,” and [10,18] “infusion or surgical therapies” as cutoff values, OPTIPARK showed good concordance with the clinician (Kw=0.73; 95% CI; 0.55,0.91) [Table 3].
Conclusions: Pending validation, OPTIPARK seems to be a viable scale of potential interest in the clinical setting to help treatment adjustment throughout the follow-up.
References: 1. Kalia LV, Lang AE. Parkinson’s disease. Lancet. 2015; 386(9996): 896-912. 2. Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, et al. Parkinson disease. Nat Rev Dis Primers. 2017, 3:17013.
To cite this abstract in AMA style:
J. Máñez Miró, F. Vivancos-Matellano, P. Martínez-Martín. Pilot study of a new tool to optimize dopaminergic treatment in Parkinson’s disease: The OPTIPARK Scale Project [abstract]. Mov Disord. 2018; 33 (suppl 2). https://www.mdsabstracts.org/abstract/pilot-study-of-a-new-tool-to-optimize-dopaminergic-treatment-in-parkinsons-disease-the-optipark-scale-project/. Accessed July 18, 2025.« Back to 2018 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/pilot-study-of-a-new-tool-to-optimize-dopaminergic-treatment-in-parkinsons-disease-the-optipark-scale-project/