Objective: To systematically modulate polyamine (PA) metabolic pathways and investigate PA biomarker findings in PD using Drosophila melanogaster over-expressing human wild-type α-synuclein gene (hSNCA). Our goal is to ascertain mechanistic relationships between PA metabolism and PD pathophysiology.
Background: PAs are organic positively-charged compounds composed of aliphatic carbon chains with multiple amino groups. PAs have key roles in various physiological processes including cellular growth and maintenance, protein and nucleic acid biosynthesis, modulation of apoptotic pathways, and neuroregulation. Our recent investigations found significant increase in the serum concentrations of three L-ornithine-derived polyamines (putrescine, spermidine, and spermine), which correlated to PD progression and certain clinical phenotypes [1]. Considering the paramount importance of polyamines and their stringent homeostatic regulation, our inquiry aims to determine if PAs can provide mechanistic insights into neurodegeneration in PD [2].
Method: This study uses a D. melanogaster model over-expressing α-synuclein (α-syn) – the pathological hallmark protein of PD. Our methodological framework integrates an array of phenotypic and molecular evaluations, assessing fly longevity, neuromotor capabilities, and biochemical indices (including protein quantification via Western blot). We will evaluate the collective and consequential impact of PAs on PD pathophysiology resulting from α-syn accumulation and aggregation.
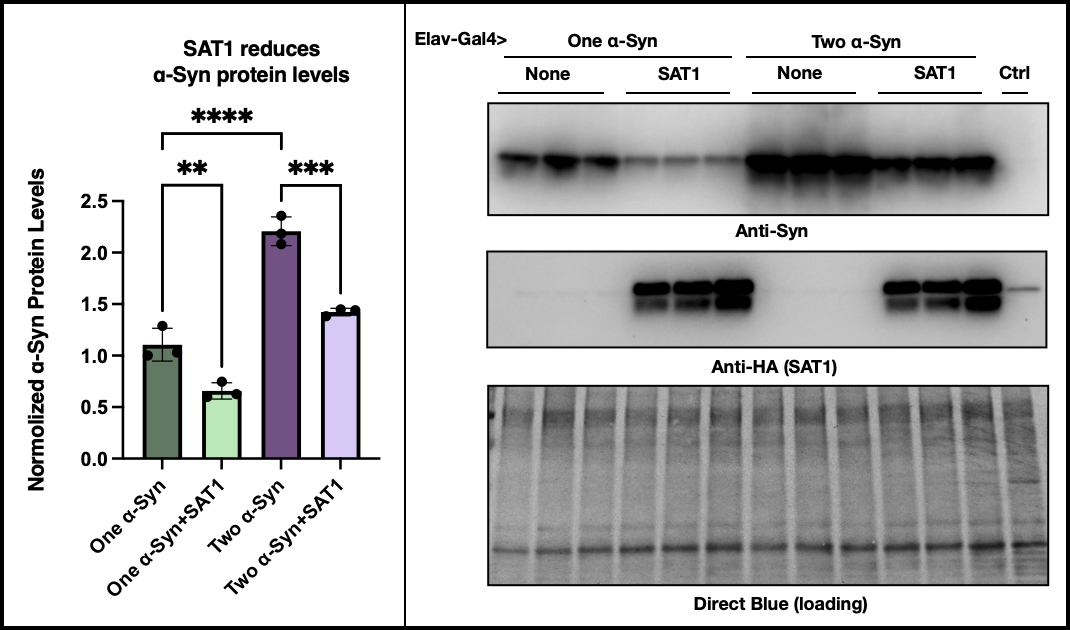
Results: Attenuating spermidine synthase (SRM) and spermine oxidase (SMOX) enzymatic activity extended fly lifespan and improved locomotor function with α-syn overexpression. α-syn protein levels decreased when SMOX was suppressed. Conversely, inhibition of spermidine/spermine N1-acetyltransferase 1 (SAT1) resulted in several adverse phenotypes. Overexpression of SAT1 significantly increased fly lifespan, while dietary administration of PAs reduced fly survival rates in α-syn-augmented flies (though it did not significantly impact locomotor activity).
Conclusion: Our findings provide new biochemical insights into a model of PD building upon biomarker findings [1,2]. These discoveries could provide guidance developing clinical trials to target specific PA pathways.
SAT1 reduces α-Syn levels in Drosophila neurons.
References: 1) LeWitt PA, et al. Polyamine biomarkers of Parkinson’s disease progression. Mov Disord 2022; 37(suppl 1):S16-S17
2) Lewandowski NM, et al. Polyamine pathway contributes to the pathogenesis of Parkinson disease. Proc Natl Acad Sc USA 2010; 107:16970-5
To cite this abstract in AMA style:
W-L. Tsou, P. Lewitt, B. Ranxhi, Z. Chbihi, Z. Bangash, S. Todi. Polyamine pathways: a promising frontier for biomarkers and therapeutic targets in Parkinson’s disease (PD) [abstract]. Mov Disord. 2024; 39 (suppl 1). https://www.mdsabstracts.org/abstract/polyamine-pathways-a-promising-frontier-for-biomarkers-and-therapeutic-targets-in-parkinsons-disease-pd/. Accessed July 4, 2025.« Back to 2024 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/polyamine-pathways-a-promising-frontier-for-biomarkers-and-therapeutic-targets-in-parkinsons-disease-pd/