Category: Other
Objective: To evaluate the real-world safety of onabotulinumtoxinA (onabotA) use over repeat treatment periods (TPs) in patients (pts) with concomitant multiple therapeutic indications.
Background: In the US, onabotA is approved for the treatment of 12 therapeutic indications. Long-term real-world safety and utilization data are limited for onabotA treatment of concomitant multiple indications.
Method: SYNCHRONIZE is a retrospective chart review study conducted at 10 US clinics, evaluating onabotA safety for ≥2 different therapeutic indications within 3-month TPs in adults. This analysis evaluated safety of onabotA for up to seven repeat TPs within 24mo.
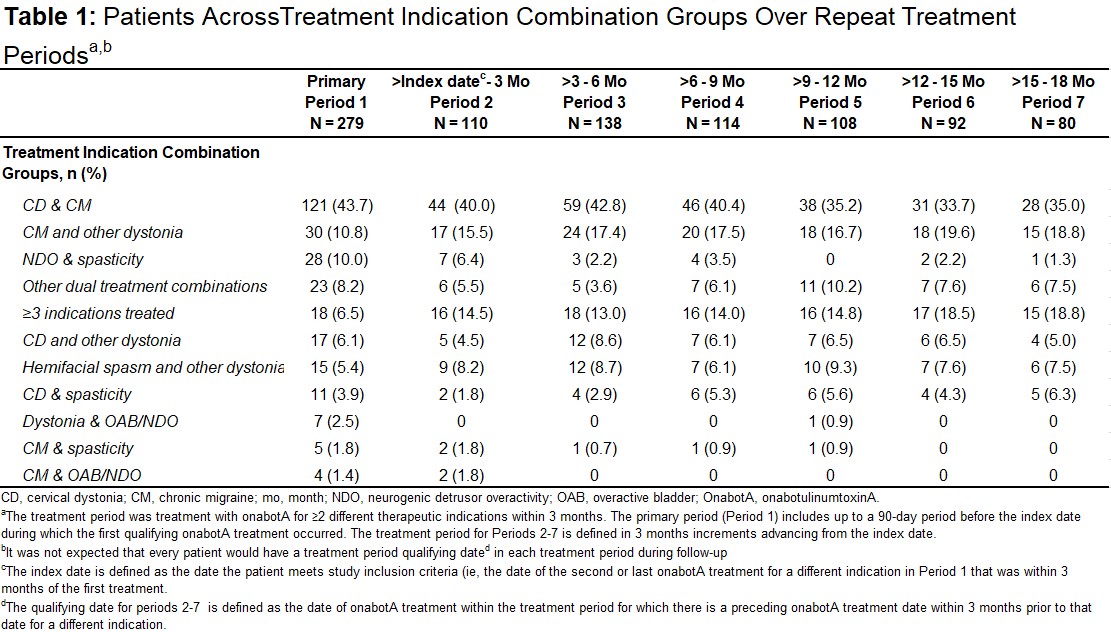
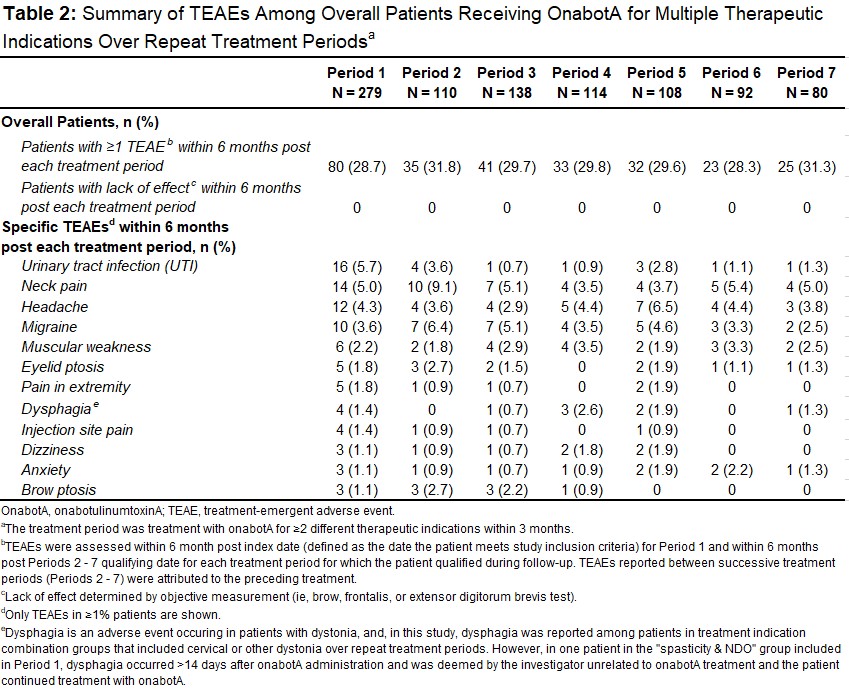
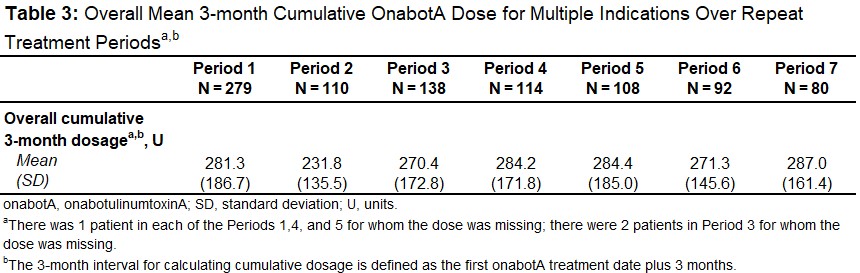
Results: A total of 279 pts were treated for ≥2 different therapeutic indications across all treatment indication combination groups analyzed (Period1; mean age, 49.2y; 79% female; 56% White) with a gradual decrease to 80 pts during the last TP (Period7) [Table1]. The overall mean onabotA treatments over the study period was 9.3 (range 2-48); the most common indications combination across all TPs was cervical dystonia and chronic migraine (range 34-44%) [Table1]. Compared to baseline, no significant change in comorbidities and concomitant medications were observed over repeat TPs. In total, 28.7% (80/279) pts reported ≥1 treatment-emergent adverse event (TEAE) after Period1; this proportion remained broadly constant after each TP; 29.7%, 29.6%, and 31.3% after Periods 3, 5 and 7, respectively [Table2]. Overall, the most common TEAEs across all TPs were UTI (range 0.7-5.7%), neck pain (range 3.7-9.1%), headache (range 2.9-6.5%), and migraine (range 2.5-6.4%) [Table2]. The majority of pts had a dosage interval (time between multiple indications treatments) of ≤24h (range 62-98%). Most pts received ≥200-<400U of cumulative 3-months onabotA dose for multiple indications (range 43-50%) with a mean total 3-month dose ranging from 232-287U [Table3]. There was no apparent trend between TEAE incidence and dosage intervals or cumulative 3-month dose over the study period. No pts were determined to have lack of effect based on clinical objective measurement.
Conclusion: OnabotA demonstrated consistent safety with no new signals observed in pts treated concomitantly for ≥2 therapeutic indications over repeat treatments up to 24mo. TEAEs were consistent with those previously reported for the individual indications treated.
Table 1
Table 2
Table 3
To cite this abstract in AMA style:
G. Forde, A. Patel, K. Martinez, A. Mayadev, B. Brucker, T. Brown, Z. Ayyoub, R. Singh, M. Nelson, A. Ukah, I. Yushmanova, S. Battucci, K. Becker Ifantides, C. Rhyne. Real-World Retrospective Safety Analysis in Patients Treated With OnabotulinumtoxinA for Multiple Therapeutic Indications Over Repeat Treatment Periods [abstract]. Mov Disord. 2024; 39 (suppl 1). https://www.mdsabstracts.org/abstract/real-world-retrospective-safety-analysis-in-patients-treated-with-onabotulinumtoxina-for-multiple-therapeutic-indications-over-repeat-treatment-periods/. Accessed July 10, 2025.« Back to 2024 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/real-world-retrospective-safety-analysis-in-patients-treated-with-onabotulinumtoxina-for-multiple-therapeutic-indications-over-repeat-treatment-periods/