Category: Parkinson’s Disease: Clinical Trials
Objective: To present the REGENERATE-PD study design: a phase 2, randomized, double-blind, surgery-controlled trial assessing efficacy and safety of intraputaminal adeno-associated virus serotype 2 containing glial cell line–derived neurotrophic factor transgene (AAV2-GDNF; AB-1005) for adults with moderate stage Parkinson’s disease (PD).
Background: A key feature of PD pathology is loss of dopaminergic neurons. GDNF is required for their development and survival and may have potential to modify the course of PD when introduced via gene therapy. In a PD phase 1b trial, intraoperative MRI-monitored AAV2-GDNF (AB-1005) delivery was safe and well tolerated. At 18 months, participants with moderate PD showed progressive functional restoration with improvement in Movement Disorder Society–Unified PD Rating Scale (MDS-UPDRS) Part III OFF score (mean [SE] ‒20.4 [4.5] pts) and normalized Good ON time (2.2 [1.0] hr) on the PD motor diary with reduced troublesome dyskinesia.
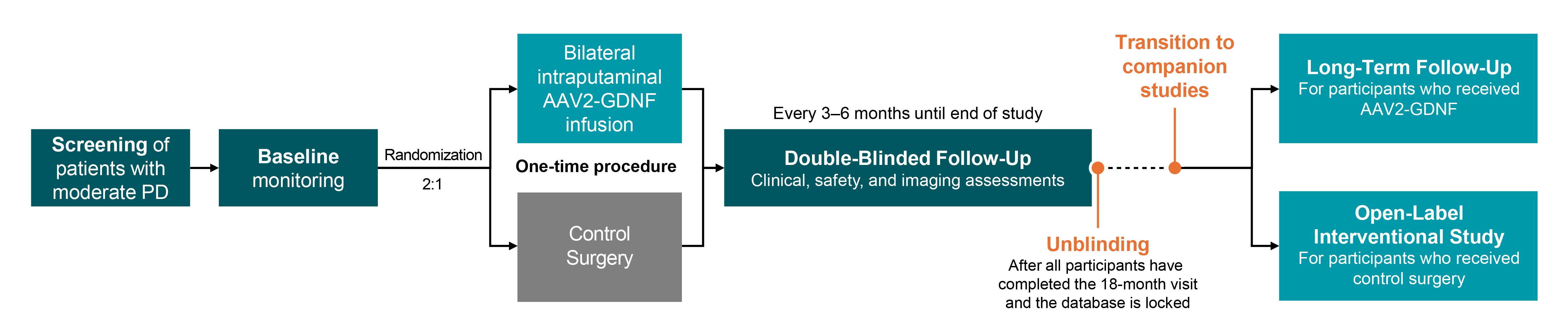
Method: REGENERATE-PD is planned to enroll 87 participants, age 45–75, with moderate PD (MDS-UPDRS Part III OFF score 33‒60) 4–10 years post-diagnosis. Participants will be randomized (2:1) to one-time bilateral infusion of AAV2-GDNF (≤1.8 mL per putamen) via an optimized neurosurgical delivery technique or control surgery (Figure). The primary objective is the efficacy of AAV2-GDNF (AB-1005) to improve/stabilize motor symptoms as assessed by change from baseline to month 18 in normalized Good ON time on the PD motor diary. Secondary endpoints include MDS-UPDRS scores (Part III OFF and ON, Part II), normalized OFF time (PD motor diary), Unified Dyskinesia Rating Scale total score, levodopa equivalent daily dose, and non-motor and quality-of-life measures. Safety will be assessed by adverse events and periodic brain MRI. Exploratory assessments include Parkinson’s Disease Comprehensive Response, 18F-DOPA uptake, and FDG PET analysis.
Results: Clinical assessments will be conducted at screening; preoperatively; and every 3–6 months post-infusion for at least 18 months (Figure). Safety will be assessed throughout the trial. The blind will be maintained until the last participant has an 18-month visit; participants will be invited to enroll in a long-term follow-up study (AAV2-GDNF arm) or open-label study (control arm).
Conclusion: The trial is expected to begin enrollment by mid-2024 (NCT06285643).
Study schema
To cite this abstract in AMA style:
A. Whone, C. Christine, M. Luz, R. Richardson, C. Urrea, N. Chhabria, C. Letourneau, M. Wisniewski, M. Fiandaca, A. van Laar, A. Kells, K. Bankiewicz. REGENERATE-PD: A Phase 2, Randomized, Double-Blind, Surgery-Controlled Study of GDNF Gene Therapy (AAV2-GDNF; AB-1005) for Moderate Stage Parkinson’s Disease [abstract]. Mov Disord. 2024; 39 (suppl 1). https://www.mdsabstracts.org/abstract/regenerate-pd-a-phase-2-randomized-double-blind-surgery-controlled-study-of-gdnf-gene-therapy-aav2-gdnf-ab-1005-for-moderate-stage-parkinsons-disease/. Accessed July 15, 2025.« Back to 2024 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/regenerate-pd-a-phase-2-randomized-double-blind-surgery-controlled-study-of-gdnf-gene-therapy-aav2-gdnf-ab-1005-for-moderate-stage-parkinsons-disease/