Objective: To report the safety and tolerability of bemdaneprocel in participants with Parkinson’s disease (PD) up to 24 months post transplantation.
Background: Bemdaneprocel is an investigational cell therapy consisting of embryonic stem cell-derived dopaminergic neuronal progenitors with preclinical studies supporting proof of concept and safety. At 12 months post transplantation, predefined safety and tolerability criteria were met and a trend towards clinical benefit was observed.
Method: In this phase 1, open-label, 24-month, non-controlled study (exPDite, NCT04802733), 12 participants with PD received a low dose (n=5; 0.9 million cells/putamen) or high dose (n=7; 2.7 million cells/putamen) of bemdaneprocel injected using a cannula bilaterally (9 deposits along 3 needle passes) into the postcommissural putamen during a single surgical session under general anesthesia. A 12-month immunosuppression regimen began intraoperatively. Treatment-emergent adverse events (TEAEs) and treatment-emergent serious adverse events (TESAEs) were collected.
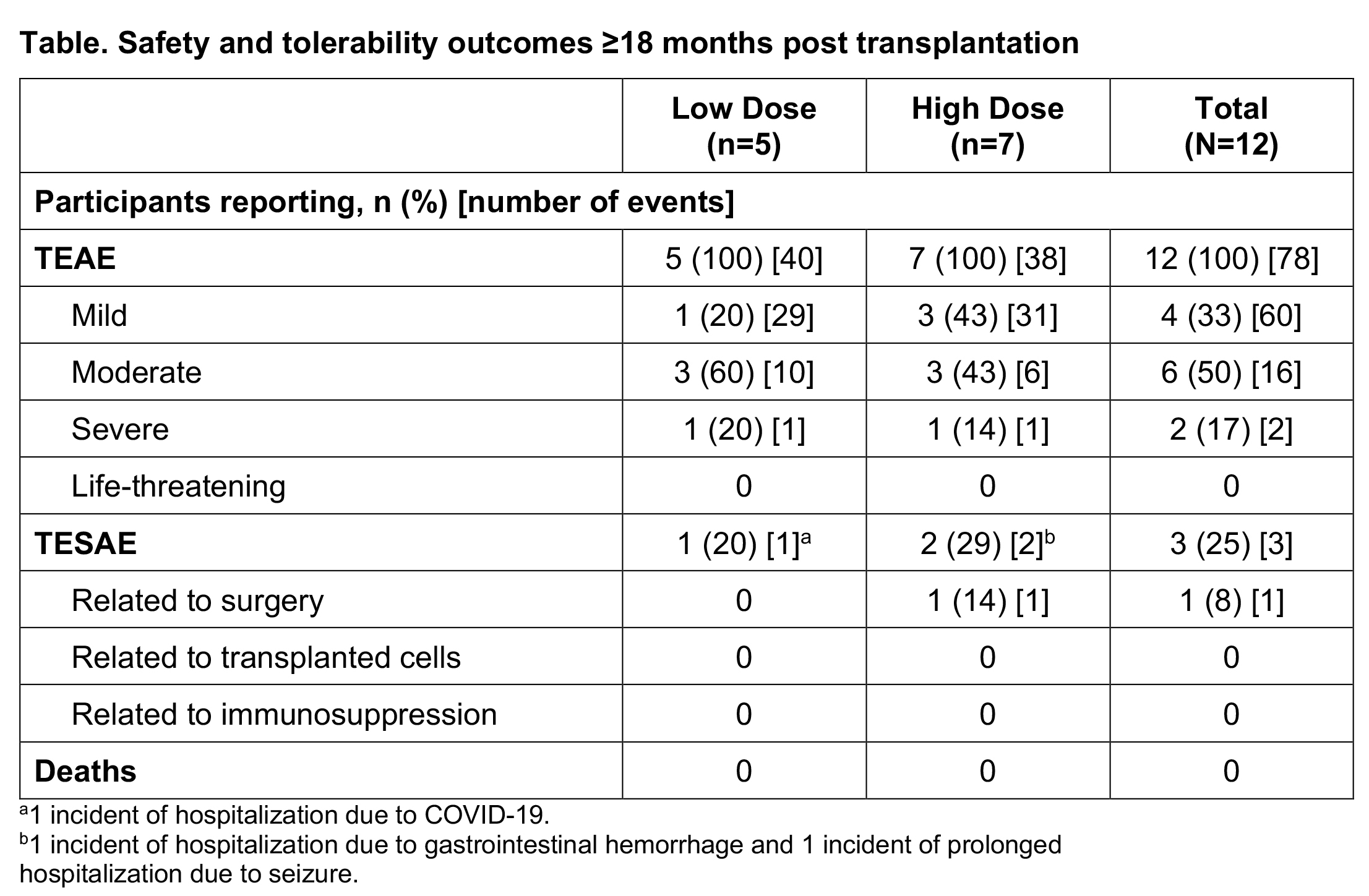
Results: Participants (N=12) were median 67.0 years of age, 75% male, and 67% White. Median (Q1, Q3) time since PD diagnosis was 9.0 (5.9, 11.5) years. At time of analysis, median (min, max) participant study duration was 21.2 (18.4, 24.6) months. Twelve participants experienced 78 TEAEs; most (76/78) were mild or moderate in severity [Table]. Three TESAEs, all unrelated to bemdaneprocel, were reported: hospitalizations due to COVID-19 (low dose) and gastrointestinal hemorrhage (high dose), and a prolonged hospitalization due to seizure 1 day after surgery (high dose) considered possibly related to surgery. No deaths, discontinuations, or graft-induced dyskinesias occurred. Feasibility of stereotactic administration was demonstrated; all participants received the intended number of cell deposits.
Conclusion: Bemdaneprocel was generally safe and well tolerated 18 months post transplantation. All participants are expected to be followed in a long-term extension study (NCT05897957) to monitor safety and durability of effect. These results support the continued development and evaluation of bemdaneprocel for the treatment of people with PD. New results from exPDite, 24 months post transplantation (12 months post discontinuation of immunosuppression), will be presented at the congress.
Table
References: 1. Piao J, Zabierowski S, Dubose BN, et al. Preclinical efficacy and safety of a human embryonic stem cell-derived midbrain dopamine progenitor product, MSK-DA01. Cell Stem Cell. 2021;28(2):217–229.
2. Kriks S, Shim JW, Piao J, et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature. 2011;480(7378):547–551.
3. Henchcliffe C, Sarva H, Lozano A, et al. Dopaminergic neuronal cell therapy for Parkinson’s disease: results from a Phase 1 study of bemdaneprocel [poster]. Presented at: International Congress of Parkinson’s Disease and Movement Disorders; August 27–31, 2023; Copenhagen, Denmark.
To cite this abstract in AMA style:
H. Sarva, C. Henchcliffe, A. Lozano, A. Fasano, S. Kalia, K. Yu, C. Brennan, W. Stemple, N. Abid, M. Yountz, A. Enayetallah, A. Lampron, V. Tabar. Safety and Tolerability of Bemdaneprocel in People With Parkinson’s Disease: Results up to 24 Months From a Phase 1 Study [abstract]. Mov Disord. 2024; 39 (suppl 1). https://www.mdsabstracts.org/abstract/safety-and-tolerability-of-bemdaneprocel-in-people-with-parkinsons-disease-results-up-to-24-months-from-a-phase-1-study/. Accessed July 9, 2025.« Back to 2024 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/safety-and-tolerability-of-bemdaneprocel-in-people-with-parkinsons-disease-results-up-to-24-months-from-a-phase-1-study/