Objective: To summarize the sleep and sedation-related safety and tolerability of pimavanserin for the treatment of Parkinson’s disease psychosis (PDP).
Background: Pimavanserin, a 5-HT2A receptor inverse agonist/antagonist, is the only FDA approved treatment for hallucinations and delusions associated with Parkinson’s disease. However, other atypical antipsychotics are frequently used off-label for PDP, such as quetiapine, and these other atypical antipsychotics may cause sedation [1, 2].
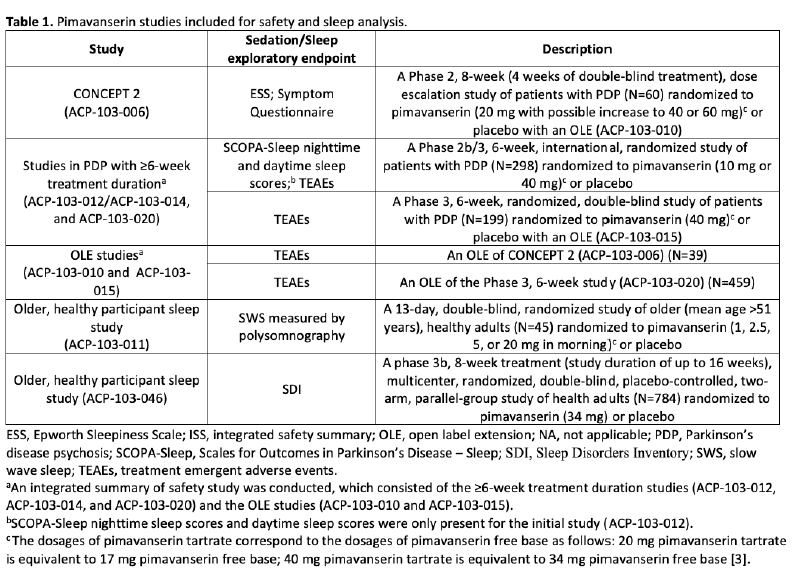
Method: Pimavanserin safety data were collected from multiple studies, all of which included treatment durations of ≥6 weeks as well as exploratory outcome measures relevant to sleep and sedation (Table 1). Studies included multiple dosages of pimavanserin and various designs (i.e., double-blind, placebo-controlled; open-label extensions; and healthy control studies). The summarized measures included outcomes of sleep, including the Epworth Sleepiness Scale (ESS) at week 4; the Scales for Outcomes in Parkinson’s Disease–Sleep (SCOPA-Sleep) at weeks 1, 2, 4, and 6; Symptom Questionnaire (poor sleeping at night) at weeks 4 and 8, slow-wave sleep (SWS) duration (a marker of sleep quality) at baseline and days 1 and 13, and the Sleep Disorders Inventory (SDI) at weeks 4 and 8. Additional safety measures included the Symptom Questionnaire (lightheadedness/dizziness) at weeks 4 and 8, and select treatment-emergent adverse events (TEAEs) in ≥2% of participants.
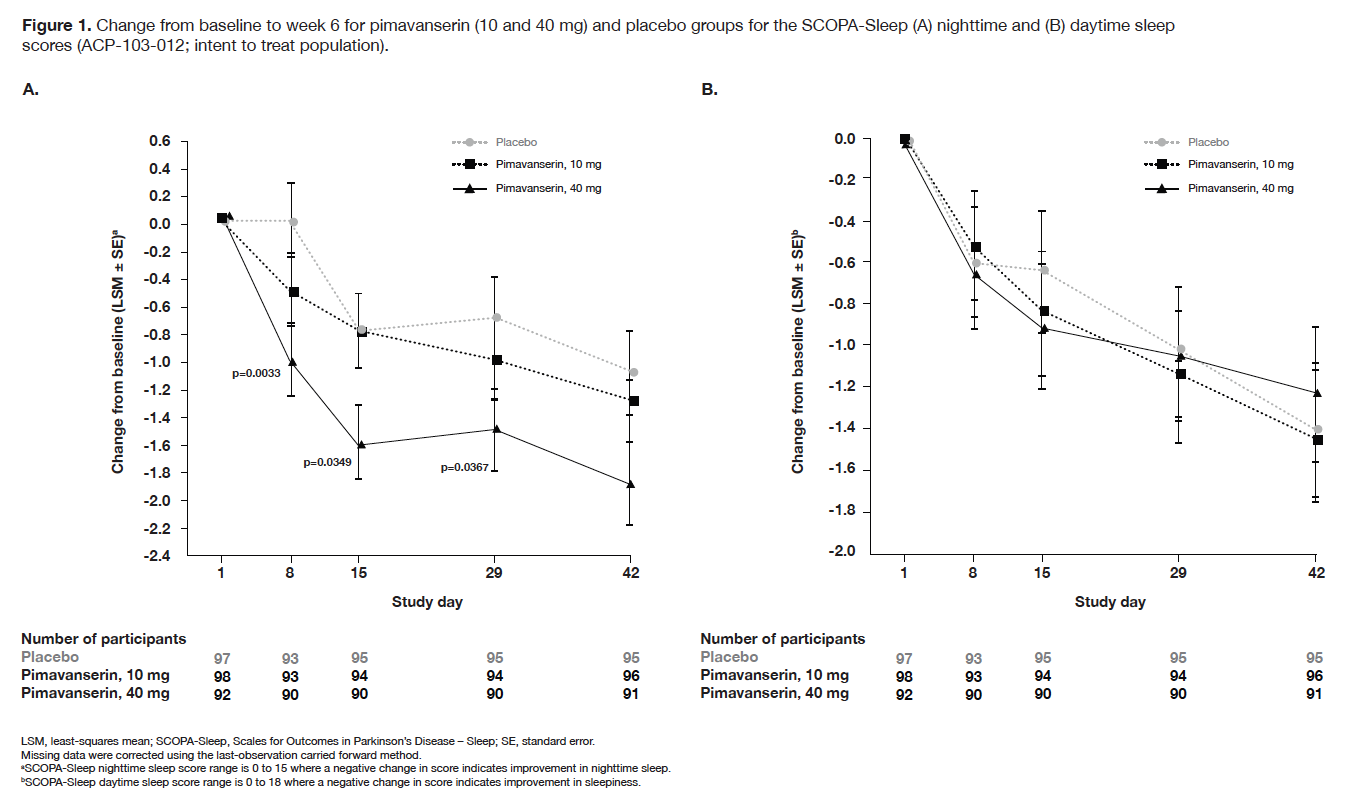
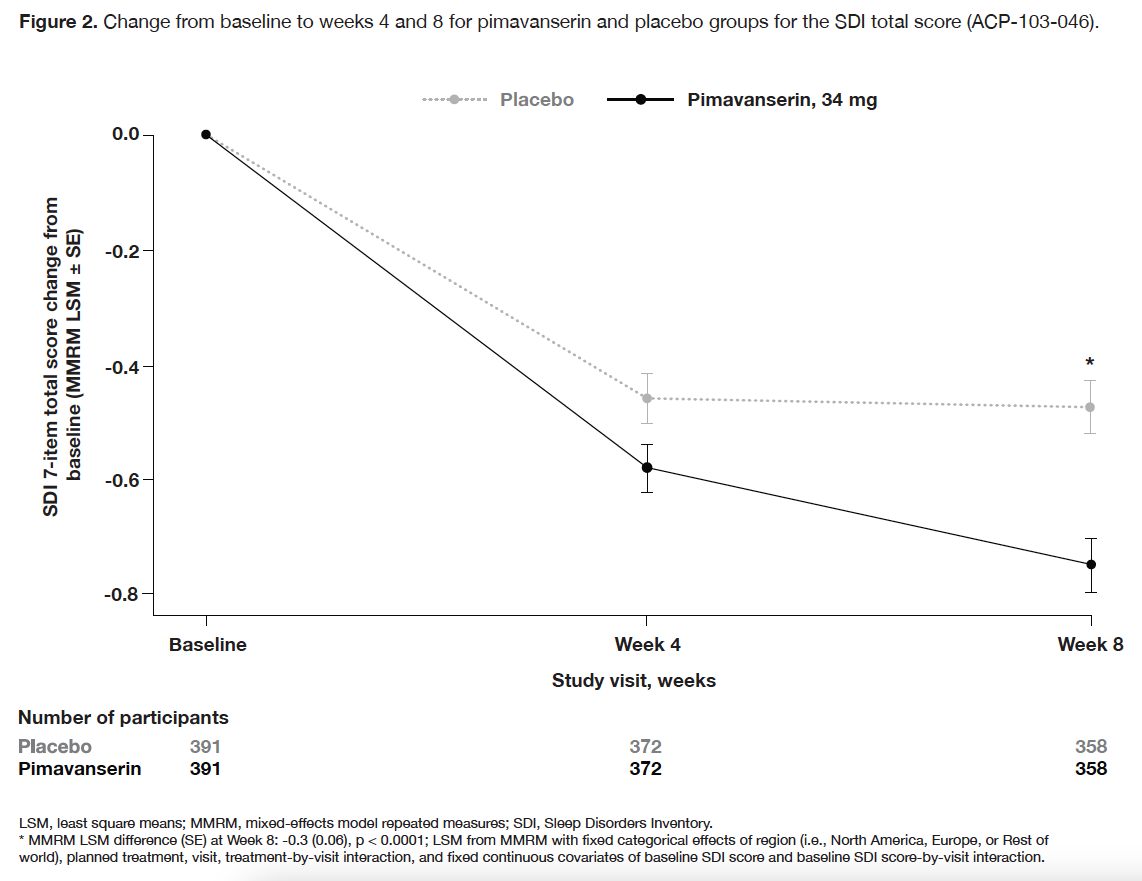
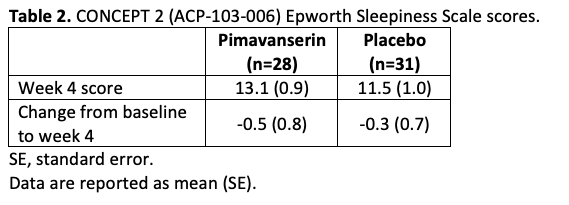
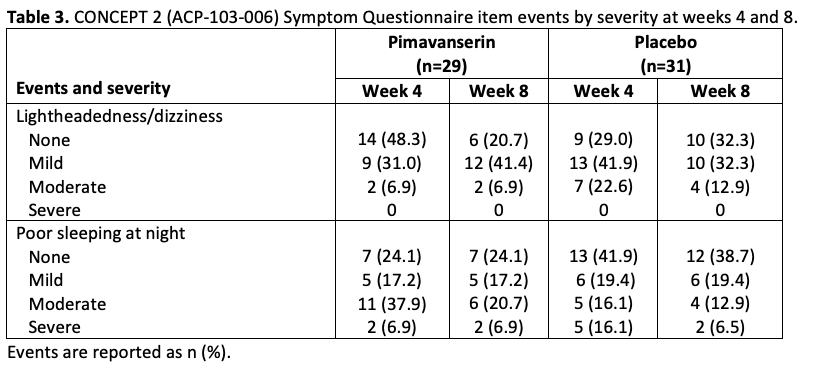
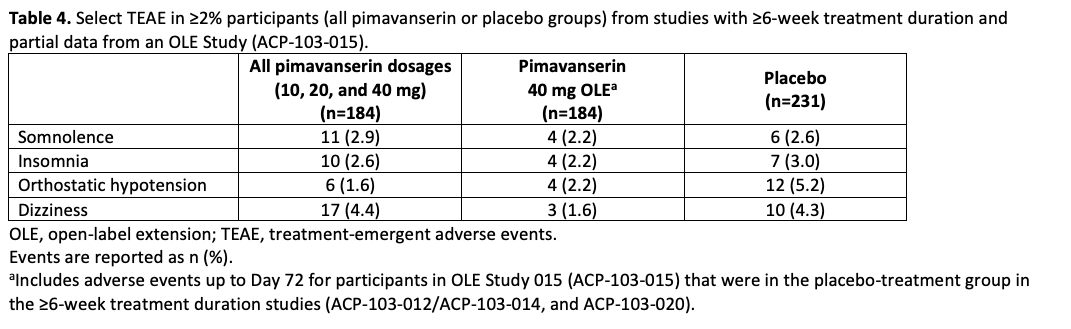
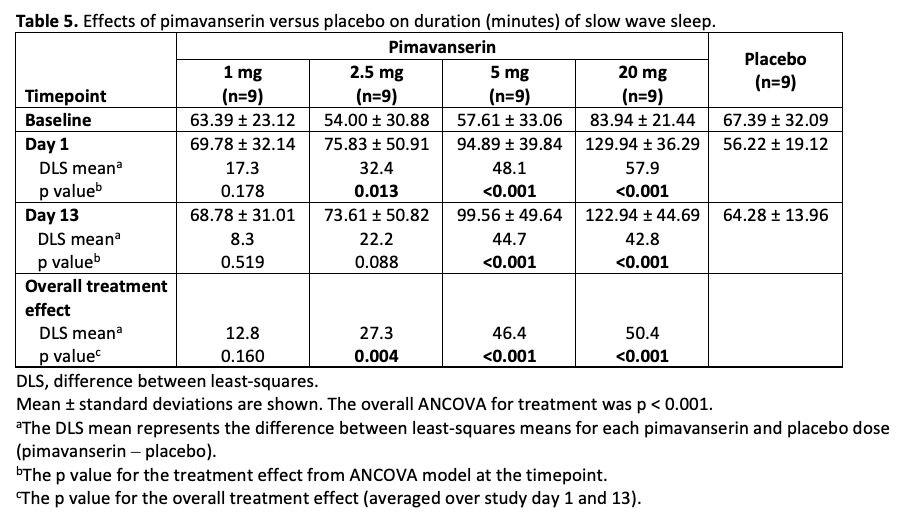
Results: In CONCEPT 2 (ACP-103-006), ESS scores (Table 2) and Symptom Questionnaire event rates (Table 3) were similar between pimavanserin and placebo groups. In study ACP-103-012, SCOPA-Sleep measurements for the pimavanserin versus placebo group were significantly improved for nighttime (Figure 1a), but not daytime (Figure 1b) sleep scores. For the ≥6-week dataset, rates of relevant TEAEs were similar between groups (Table 4). For the healthy volunteer studies (ACP-103-011 and ACP-103-046), pimavanserin was associated with increases in SWS duration (Table 5) and SDI by week 8 (Figure 2), respectively.
Conclusion: These data suggest that pimavanserin may be associated with low levels of sedation and other sleep-related adverse events as well as improvements in nighttime sleep and sleep architecture. These observations should be further evaluated with randomized controlled trials.
Figure 1
Figure 2
Table 1
Table 2
Table 3
Table 4
Table 5
References: [1] Miller, D.D. Atypical antipsychotics: Sleep, sedation, and efficacy. Prim Care Companion J Clin Psychiatry. 2004;6[suppl 2]:3–7.
[2] Shotbolt, P., et al. Quetiapine in the treatment of psychosis in
Parkinson’s disease. Ther Adv Neurol Disord. 2010;3(6)339–350.
[3] Cruz, M.P. Pimavanserin (Nuplazid): A treatment for hallucinations and delusions associated with Parkinson’s disease. P T. 2017;42(6):368–371.
To cite this abstract in AMA style:
A. Berrio, L. Chrones, V. Abler, R. Hauser. Safety of Pimavanserin for Parkinson’s Disease Psychosis: Exploratory Analysis of Sedation and Sleep Data From Clinical Studies [abstract]. Mov Disord. 2024; 39 (suppl 1). https://www.mdsabstracts.org/abstract/safety-of-pimavanserin-for-parkinsons-disease-psychosis-exploratory-analysis-of-sedation-and-sleep-data-from-clinical-studies/. Accessed July 3, 2025.« Back to 2024 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/safety-of-pimavanserin-for-parkinsons-disease-psychosis-exploratory-analysis-of-sedation-and-sleep-data-from-clinical-studies/