Session Information
Date: Wednesday, September 25, 2019
Session Title: Non-Motor Symptoms
Session Time: 1:15pm-2:45pm
Location: Agora 3 West, Level 3
Objective: To investigate in vivobrainstem structural connectivity changes in a model of premanifest synucleinopathy, REM sleep behavior disorder (RBD), using 7 T high spatial-resolution diffusion-tensor-imaging (DTI) and a recently developed brainstem nuclei atlas [1-2].
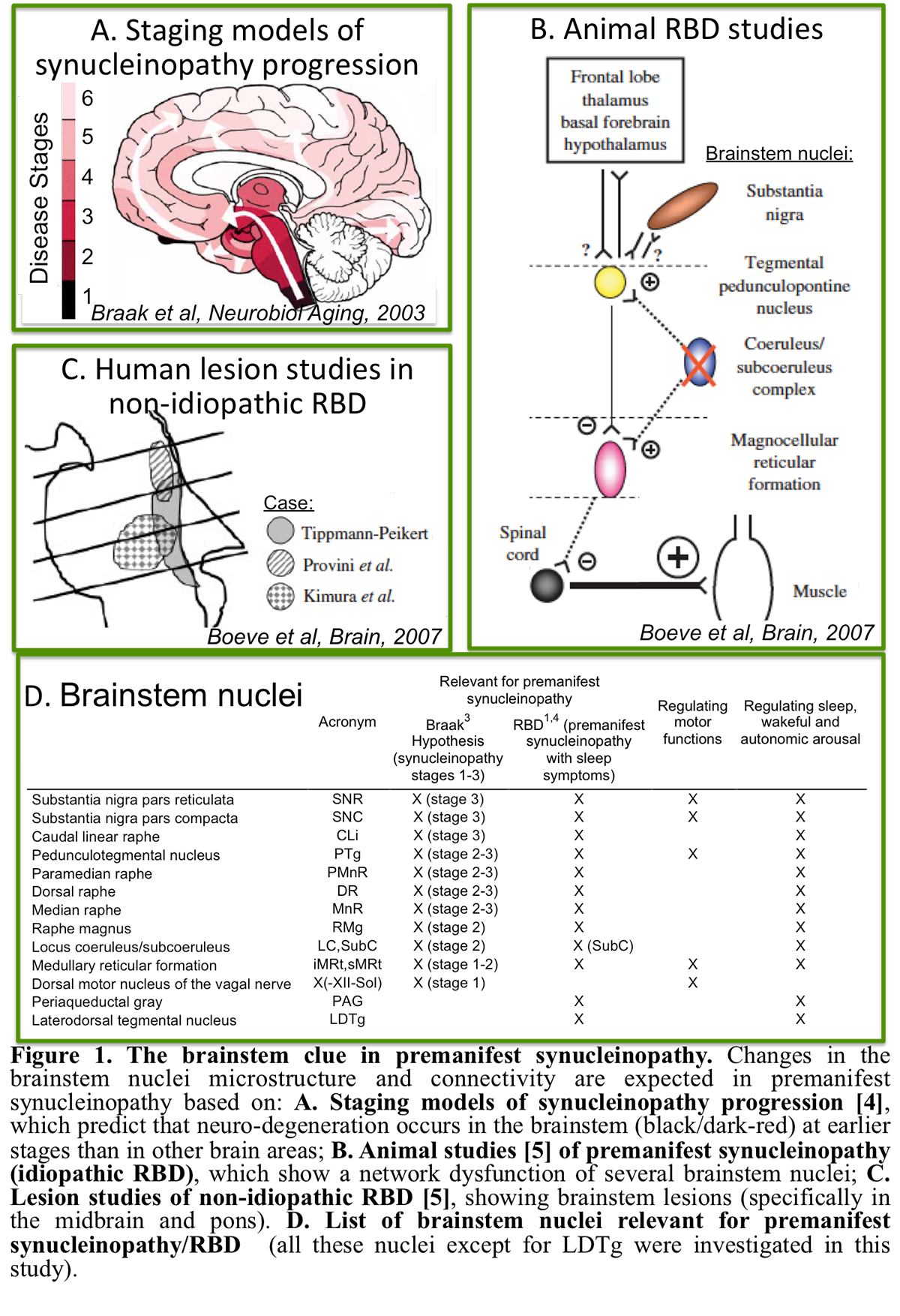
Background: RBD patients show a loss of muscle atonia during REM sleep, and have up to a 90% risk of developing a neurodegenerative synucleinopathy 14years post RBD-diagnosis [3]. Changes in brainstem nuclei microstructure and connectivity are expected in RBD/premanifest synucleinopathy based on animal studies and ex-vivo human studies [4-5] [figure1]. Yet, these brainstem changes underlying RBD/premanifest-synucleinopathy are understudied in living humans.
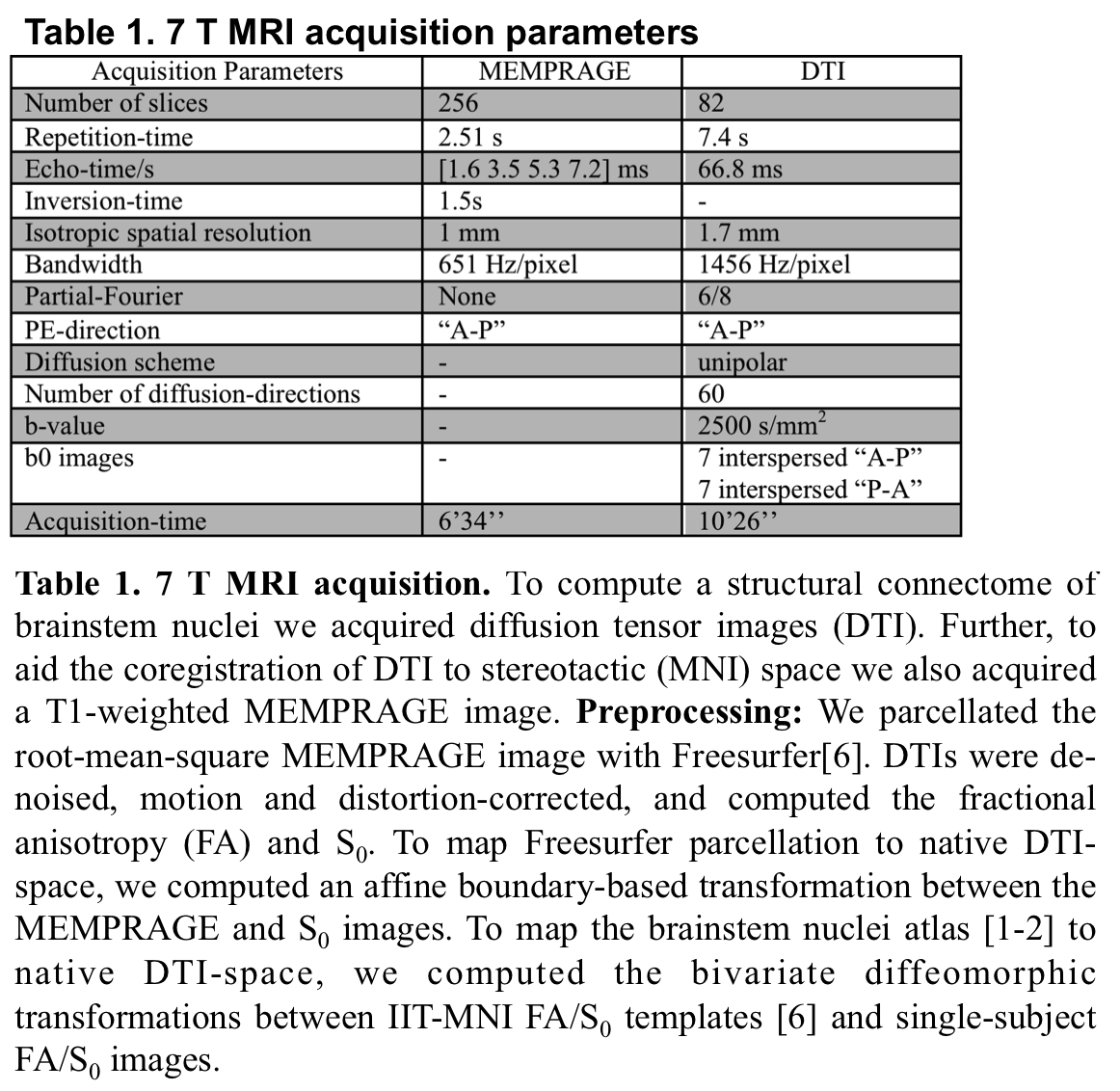
Method: Data acquisition:Under IRB-approval, we acquired at 7 T a MEMPRAGE and a spin-echo DTI[table1],in 10 idiopathic-RBD patients (age: 69.7±1.59 yrs) and 7 controls (age: 67.6±2.6 yrs). Data analysis:a) Preprocessing:see Table1. b) Definition of seed and target regions for DTI-based connectivity analysis:As seed regions, we used structural probabilistic atlas labels of 9 brainstem nuclei [1-2] and non-probabilistic labels of 5 brainstem nuclei [figure1]. As target regions, we used labels of 47 brainstem nuclei [1-2], 41 cortical/subcortical bilateral regions from Freesurfer parcellation [7], hypothalamus [8] and ventral/dorsal spinal cord.c) Single-subject and group DTI-based connectivity analysis:We run deterministic tractography [9]propagating 10,000 streamlines from each seed and computed a structural “connectivity-index” (range: [0 1]) for each pair of seed-target masks (= fraction of streamlines propagated from seed reaching target).Then, we averaged the connectivity-index of brainstem nuclei with target-regions across subjects and displayed the group connectome with a 2D circular diagram [10].
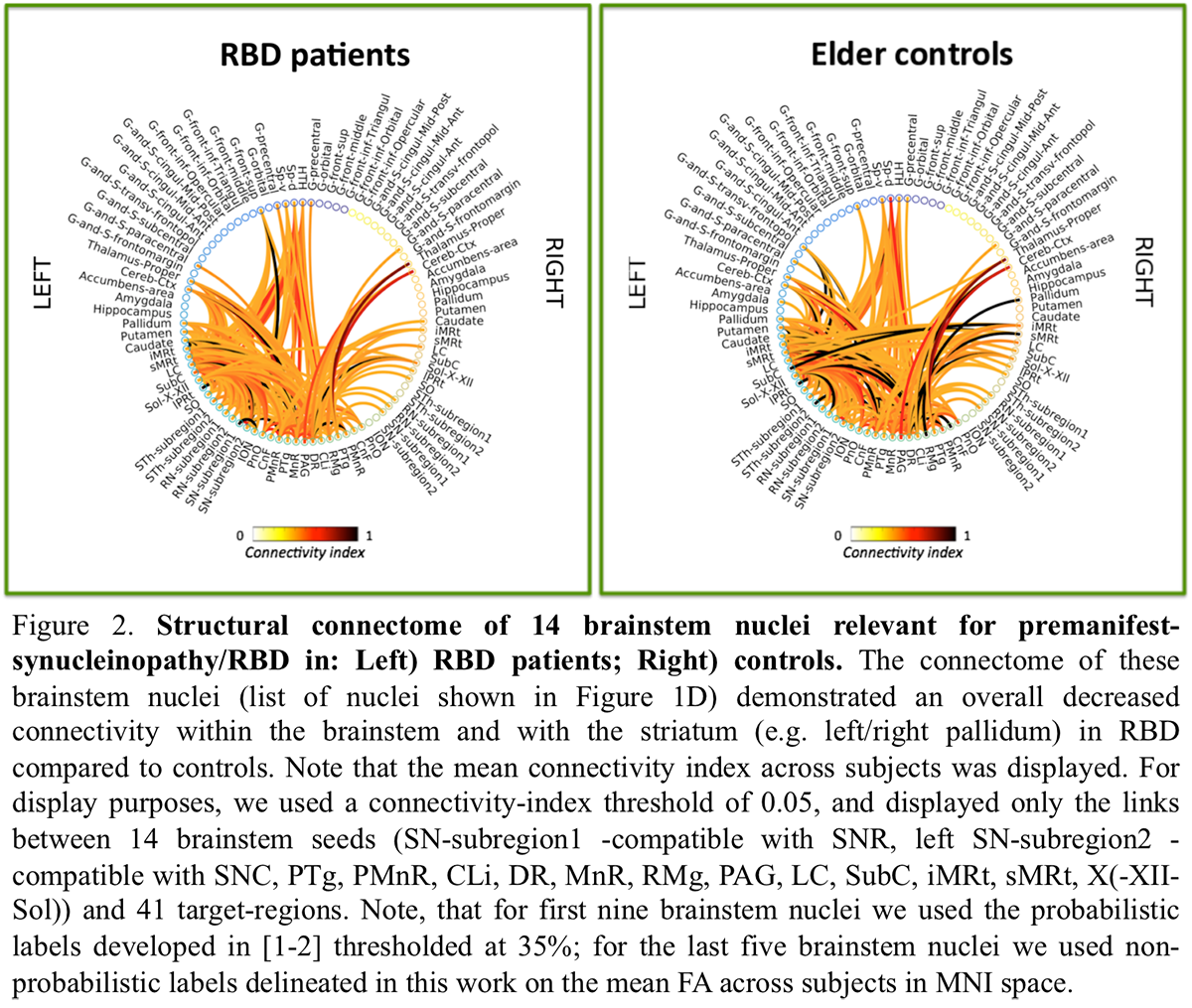
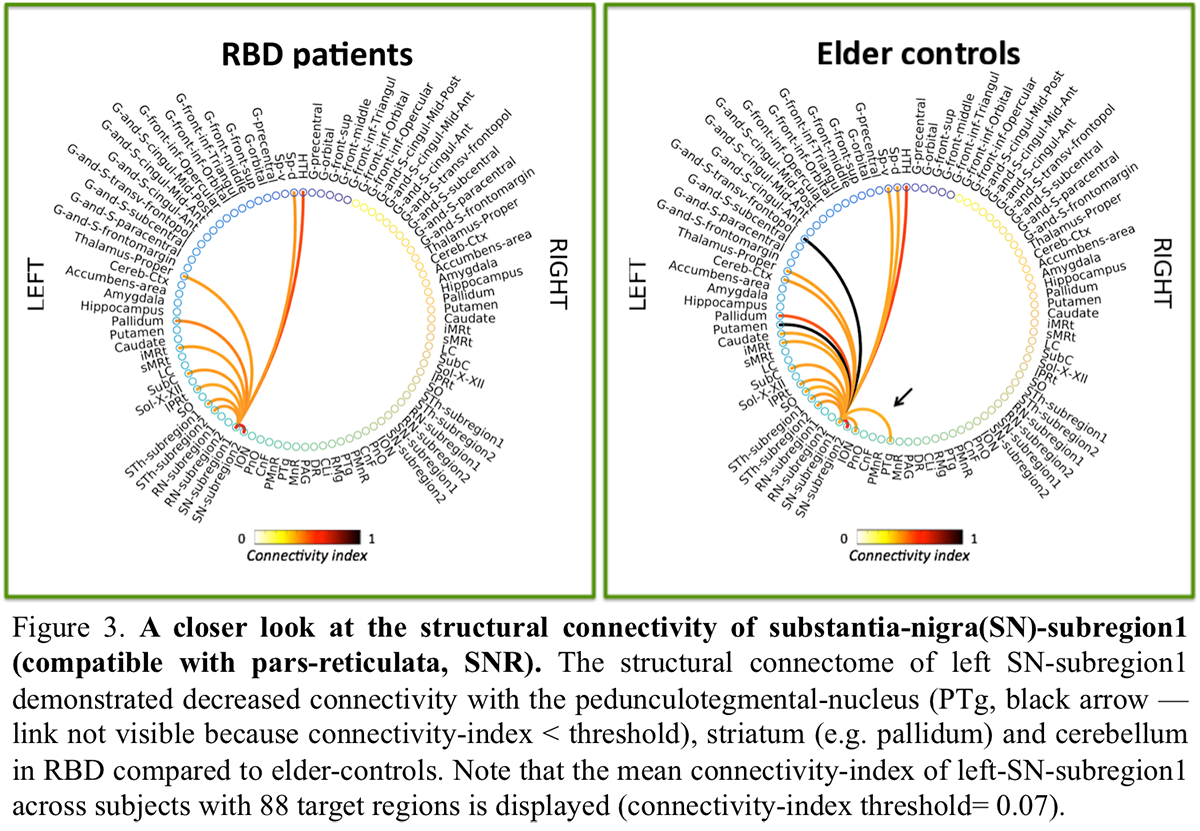
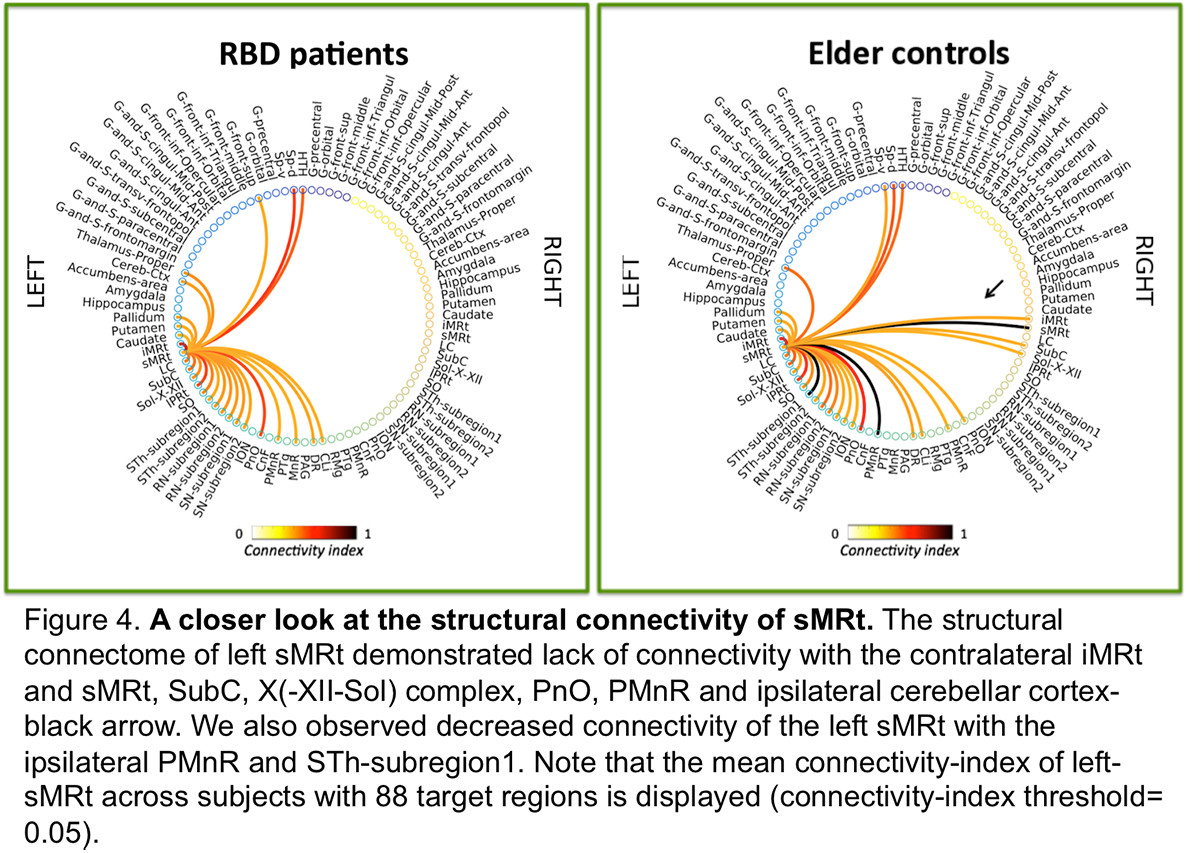
Results: The structural connectome of brainstem nuclei showed overall decreased connectivity of brainstem nuclei in RBD compared to controls [figure2]. Specifically, the observeddecreased connectivity of SNR with the PTgand of sMRt [figure3-4] with contralateral brainstem nuclei in RBD is in line with animal RBD-studies and models of synucleinopathy progression [5].
Conclusion: The structural connectome of brainstem pathways in living humans is a promising tool to better understand and assess premanifest synucleinopathy stages.
References: 1. Bianciardi M, Toschi N, Edlow BE, et al., (2015), ‘Toward an in vivo neuroimaging template of human brainstem nuclei of the ascending arousal, autonomic, and motor systems’. Brain Connect, vol. 5, no. 10, pp. 597-607. 2. Bianciardi M, Strong C, Toschi N, et al., (2018), ‘A probabilistic template of human mesopontine tegmental nuclei from in vivo 7T MRI’. Neuroimage, vol. 170, pp. 222-230. 3. Postuma RB. (2014), ‘Prodromal Parkinson’s disease – using REM sleep behavior disorder as a window’. Parkinsonism Relat Disord, vol. 20, Suppl1: S1-4. 4. Braak H, Del Tredici K, Rub U, et al., (2003), ‘Staging of brain pathology related to sporadic Parkinson’s disease’. Neurobiol Aging, vol. 24, no. 2, pp. 197-211. 5. Boeve BF, Silber MH, Saper CB, et al., (2007), ‘Pathophysiology of REM sleep behavior disorder and relevance to neurodegenerative disease’. Brain, vol. 130, no. Pt 11, pp. 2770-2788. 6. Varentsova A, Zhang S, Arfanakis K., (2014), ‘Development of a high angular resolution diffusion imaging human brain template’. Neuroimage, vol. 91, pp. 177-186. 7. Desikan RS, Segonne F, Fischl B, et al. (2006), ‘An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest’. Neuroimage, vol. 31, no. 3, pp. 968-980. 8. Pauli WM, Nili AN, Tyszka M. (2018) ‘Data Descriptor: A high-resolution probabilistic in vivo atlas of human subcortical brain nuclei’. Scientific Data, vol. 5, 180063. 9. Basser PJ, Pajevic S, Pierpaoli C, et al. (2000), ‘In vivo fiber tractography using DT-MRI data’. Magnetic Resonance in Medicine, vol. 44, 625-632. 10. Irimia A, Chambers MC, Torgerson CM, et al., (2012), ‘Circular representation of human cortical networks for subject and population-level connectomic visualization’. Neuroimage, vol. 60, no. 2, pp. 1340-1351.
To cite this abstract in AMA style:
MG. García-Gomar, LD. Lewis, L. Wald, B. Rosen, A. Videnovic, M. Bianciardi. Structural connectome of brainstem nuclei in REM sleep behavior disorder, a model of premanifest synucleinopathy [abstract]. Mov Disord. 2019; 34 (suppl 2). https://www.mdsabstracts.org/abstract/structural-connectome-of-brainstem-nuclei-in-rem-sleep-behavior-disorder-a-model-of-premanifest-synucleinopathy/. Accessed February 7, 2026.« Back to 2019 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/structural-connectome-of-brainstem-nuclei-in-rem-sleep-behavior-disorder-a-model-of-premanifest-synucleinopathy/