Objective: We aim to study the combined effect of the GBA1 p.N409S mutation and a complete loss of Parkin based on a patient-based stem cell model of a carrier of a heterozygous GBA1 p.N409S mutation and a homozygous deletion of PARK2 exon 3.
Background: Variants in the GBA1 gene encoding the lysosomal enzyme β-glucocerebrosidase (Gcase) are the most common genetic risk factor for developing Parkinson’s disease (PD)[1]. Homozygous deletions in the Parkin gene (PARK2) have been associated with autosomal recessive forms of PD[2].
Method: Midbrain dopaminergic neurons were generated as described previously[3]. Enzymatic activity measurements within the lysosomal fraction were performed using a fluorometric readout[4]. Mechanistic pathways were studied with inhibitors of the proteasome (MG132) and of translation (Cycloheximide). Alpha-synuclein protein levels were analyzed by Western Blot.
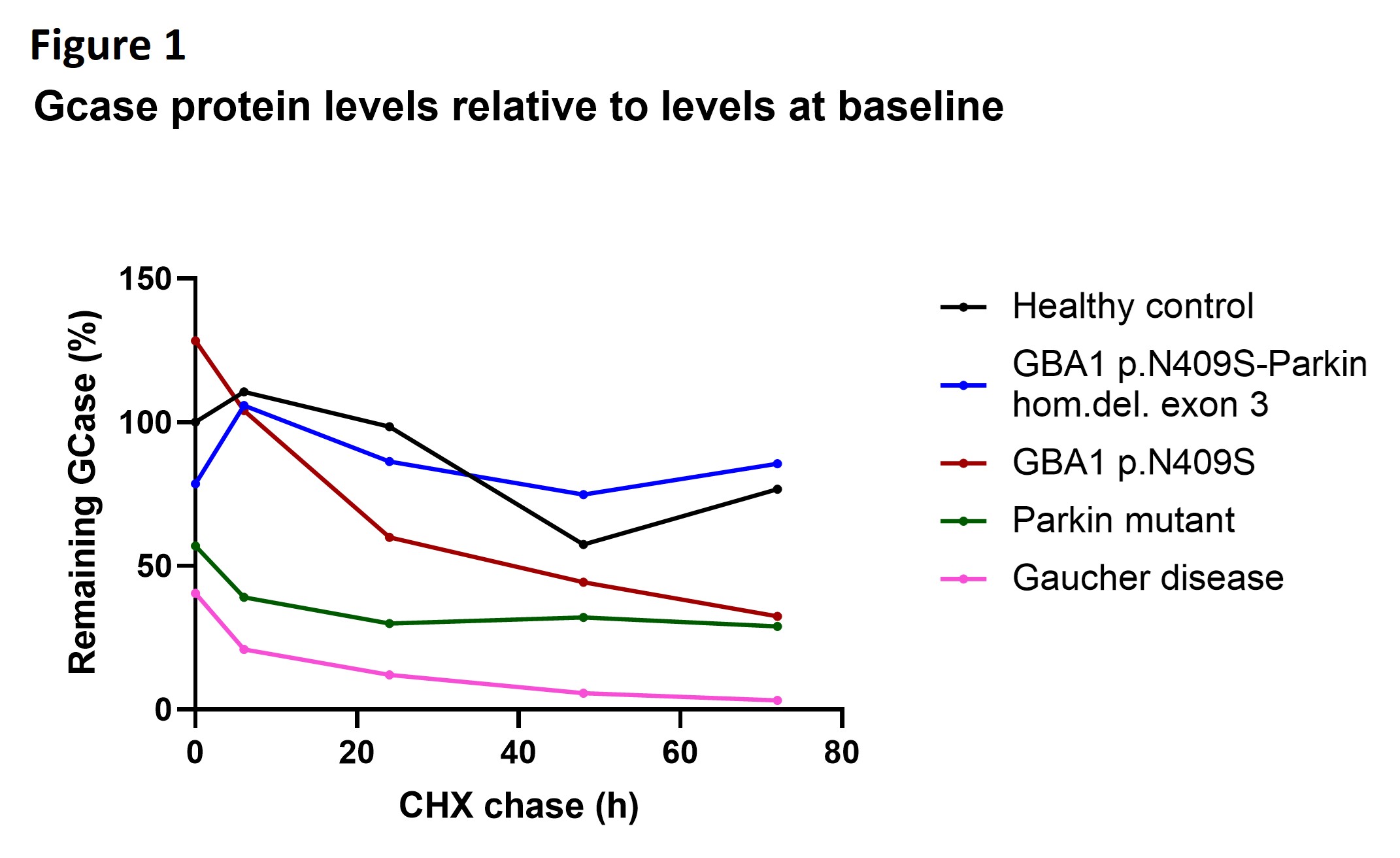
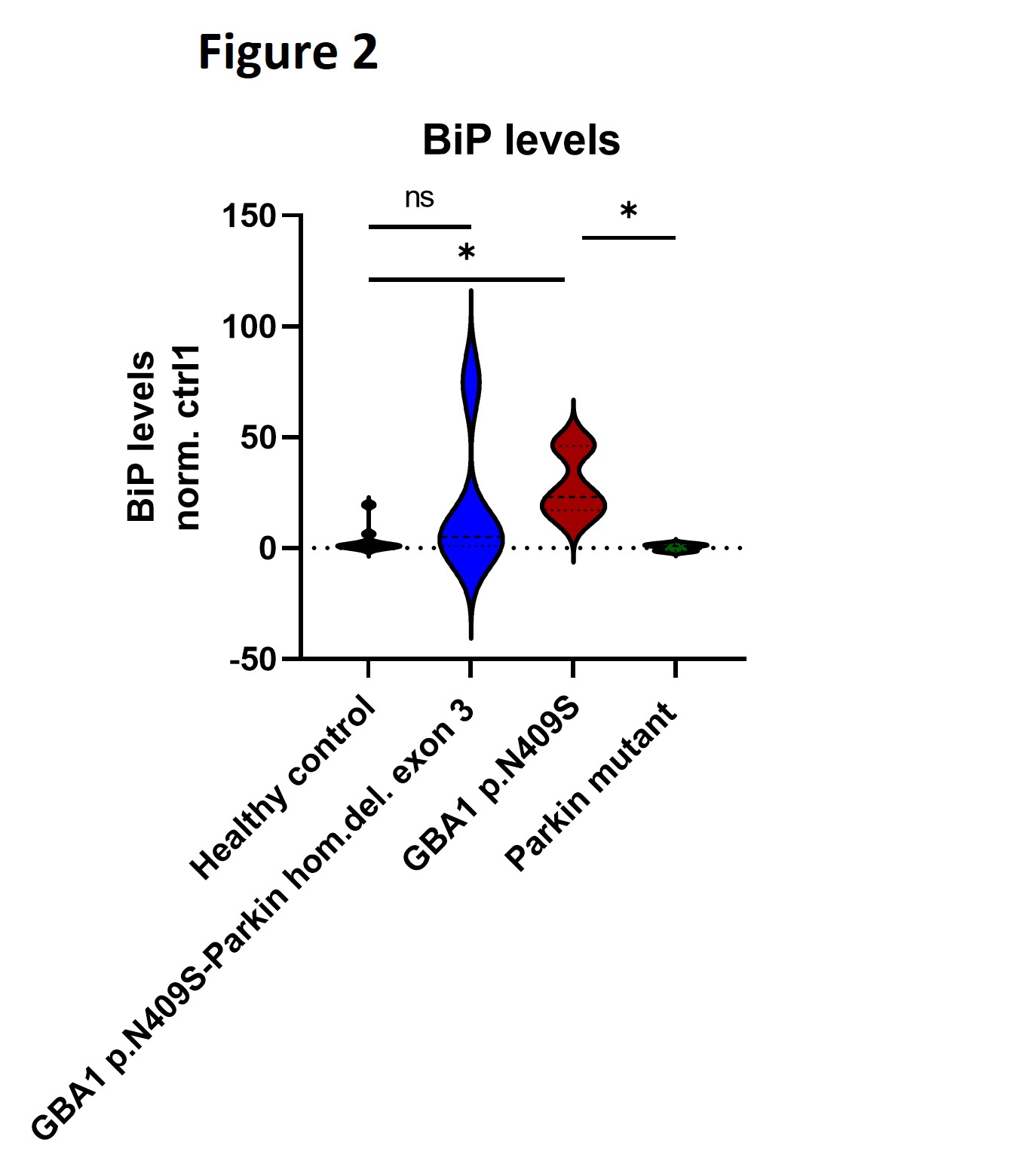
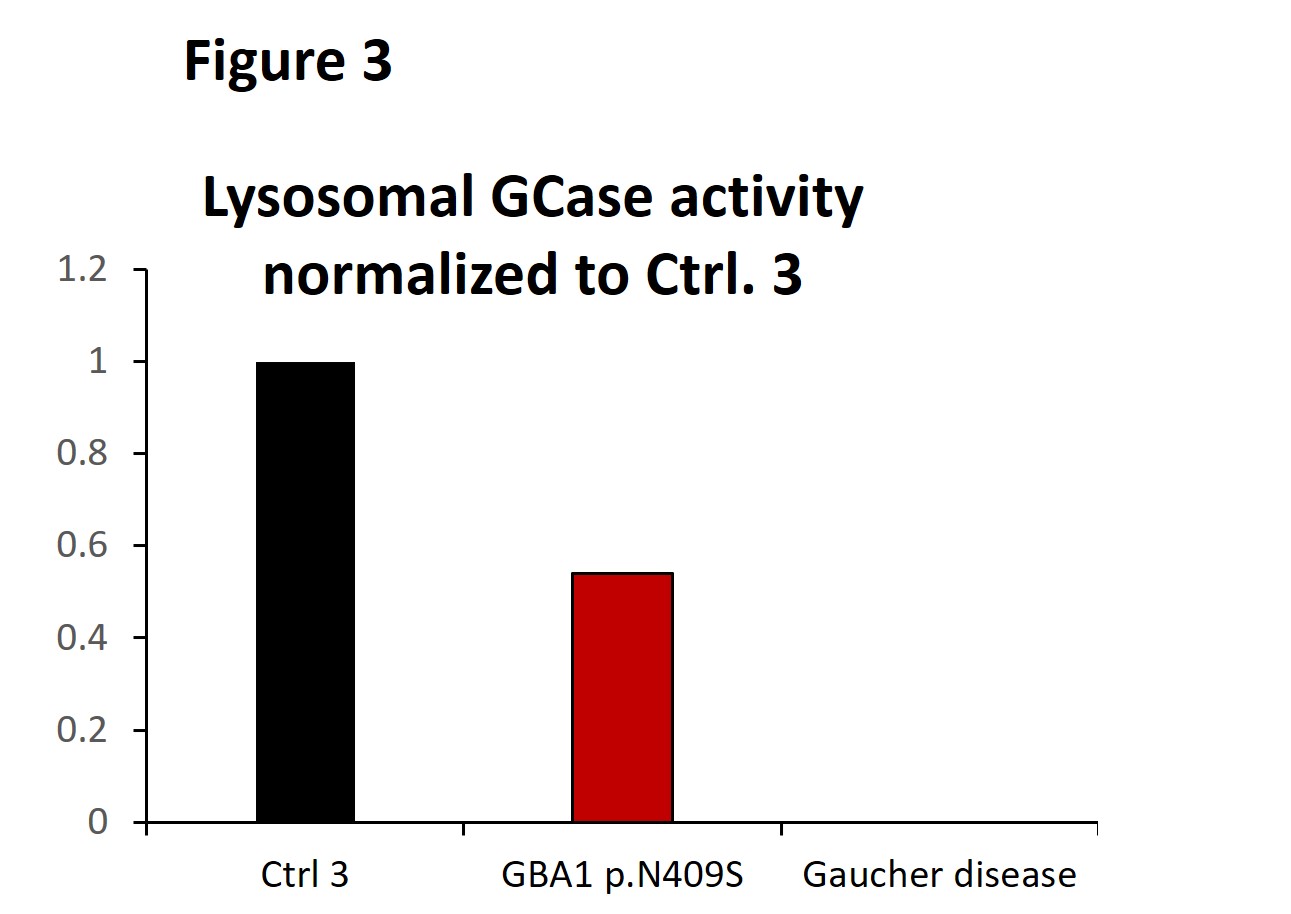
Results: Our results revealed an epistatic interaction between PARK2 and GBA1. In contrast to what is expected in GBA1 mutation carriers, lower intracellular monomeric α-synuclein protein levels were observed in the double mutation carrier compared to healthy controls. Proteasomal inhibition in the nerve cells led to increased Gcase protein levels in GBA1 p.N409S single mutants only. Moreover, our data suggest that GCase stability is the lowest in the presence of mutated GCase species and wild-type Parkin compared to mutated Gcase in the absence of Parkin and compared to healthy controls [figure1]. In addition, we found that protein levels of the endoplasmic reticulum (ER) chaperone BiP that becomes activated upon ER stress were significantly increased in GBA1 p.N409S single mutants and not in GBA-Parkin double mutation carriers [figure2]. Lastly, we found a decrease of approximately 50% of lysosomal Gcase activity in mutant GBA1 p.N409S midbrain dopaminergic neurons compared to healthy controls [figure3].
Conclusion: Mutant Gcase protein could be protected from proteasomal degradation in neurons when co-occuring with loss of Parkin compared to GBA1 p.N409S carrying neurons with normal Parkin levels. Increased levels of even mutant Gcase could be beneficial and potentially lead to a decrease in α-synuclein protein to a level similar to control. If so, Parkin inhibitors could be a potential option to treat GBA-PD at least due to p.N409S mutations.
Figure 2: CHX chase
Figure 2: BiP protein levels
Figure 3: Lysosomal GCase enzymatic activity
References: [1] Smith, L., & Schapira, A.H.V. (2022). GBA Variants and Parkinson Disease: Mechanisms and Treatments. Cells, 11(8):1261.
[2] Mizuno, Hattori, N., Mori, H., Suzuki, T., & Tanaka, K. (2001). Parkin and Parkinson’s disease. Curr Opin Neurol., 14(4), 477-82.
[3] Reinhardt, P., Glatza, M., Hemmer, K., Tsytsyura, Y., Thiel, C.S:, Höing, S., …, & Sterneckert, J. (2013). Derivation and expansion using only small molecules of human neural progenitors for neurodegenerative disease modeling. PloS One, 8(3): e59252.
[4] Marshall, J., McEachern, K.A., Cavanagh Kyros, J.A., Nietupski, J.B., Budzinski, T.L., Ziegler, R.J. (2002). Demonstration of Feasibility of In Vivo Gene Therapy for Gaucher Disease Using a Chemically Induced Mouse Model. Molecular Therapy, 6(2), 179-189.
To cite this abstract in AMA style:
C. Oleksy, Z. Hanss, F. Massart, G. Arena, I. Boussaad, R. Krüger. Studying digenic Parkinson’s disease in a stem cell model carrying mutant p.N409S in the GBA1 gene and the homozygous deletion of exon 3 PARK2 [abstract]. Mov Disord. 2024; 39 (suppl 1). https://www.mdsabstracts.org/abstract/studying-digenic-parkinsons-disease-in-a-stem-cell-model-carrying-mutant-p-n409s-in-the-gba1-gene-and-the-homozygous-deletion-of-exon-3-park2/. Accessed July 14, 2025.« Back to 2024 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/studying-digenic-parkinsons-disease-in-a-stem-cell-model-carrying-mutant-p-n409s-in-the-gba1-gene-and-the-homozygous-deletion-of-exon-3-park2/