Session Information
Date: Saturday, October 6, 2018
Session Title: Parkinson’s Disease: Clinical Trials, Pharmacology And Treatment
Session Time: 1:45pm-3:15pm
Location: Hall 3FG
Objective: Describe the design and baseline characteristics of a long-term (1 year) study of 2 ND0612 dose regimens, with emphasis on systemic and local safety profile.
Background: ND0612 is a levodopa/carbidopa (LD/CD) solution continuously administered via subcutaneous infusion. Efficacy results from Phase-2 studies have shown significant reductions in total daily OFF time accompanied by improvements in good ON time (i.e. without troublesome dyskinesia) and overall clinical status.
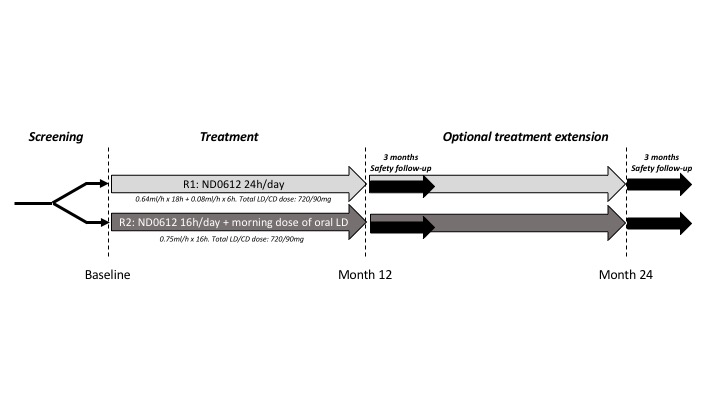
Methods: BeyoND is an ongoing multi-center, international, open-label, safety study of ND0612 for the management of motor fluctuations in PD. Adult PD patients (Hoehn & Yahr ≤3 during ON) taking ≥4 LD doses/day and with ≥2 hours of OFF time per day including predictable early morning OFF periods were eligible for the study. Patients had to be taking ≥1 other PD medication (or have attempted to take ≥30 days in prior year). The study design in shown in [figure1]. Patients were assigned to open-label treatment with high dose ND0612 for either 24 hours ‘round the clock’ infusion, or for 16 hours plus a morning dose of oral LD to a total LD/CD daily dose of 720/90mg. Adjunct oral PD medications could be taken as needed. Safety and tolerability is assessed through adverse event reporting (with a focus on infusion site reactions) and percentage of early treatment discontinuations.
Results: By October 2017, 162 patients had been enrolled (24-hour regimen: n=90; 16-hour regimen: n=72) at 48 sites in 8 countries. Overall, 65% of patients were male and the mean ±SD age was 64. 5 ±8.67 years. The mean ±SD time since PD diagnosis was 9.5 ±4.9 years and the mean ±SD duration of motor fluctuations was 5.6 ±4.4 years. The mean ±SD LD dose was 1017 ±572mg. The mean duration of daily OFF time at baseline was 5.5 ±2.8 hours and the mean baseline UPDRS motor score was 28.9 ±12.8.
Conclusions: This will be the first study to evaluate the long-term safety of high dose ND0612 in patients with motor fluctuations not adequately controlled with oral therapies.
To cite this abstract in AMA style:
W. Poewe, F. Stocchi, A. Espay, T. Rachmilewitz Minei, S. Oren, R. Case, K. Kieburtz, C. Olanow. The BeyoND study: Design and baseline characteristics of a study evaluating the long-term safety of ND0612 for motor fluctuations in Parkinson’s disease [abstract]. Mov Disord. 2018; 33 (suppl 2). https://www.mdsabstracts.org/abstract/the-beyond-study-design-and-baseline-characteristics-of-a-study-evaluating-the-long-term-safety-of-nd0612-for-motor-fluctuations-in-parkinsons-disease/. Accessed July 12, 2025.« Back to 2018 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/the-beyond-study-design-and-baseline-characteristics-of-a-study-evaluating-the-long-term-safety-of-nd0612-for-motor-fluctuations-in-parkinsons-disease/