Objective: To compare the early results of the introduction of MSCs via different routs of administration on the dynamics of the motor symptoms in patients with Parkinson’s disease (PD).
Background: PD is a progressive neurodegenerative disease. The use of mesenchymal stem cells (MSCs) is a perspective method to influence on the pathogenesis of the disease.
Method: MSCs were transplanted to patients with PD via single intranasal or intravenous injection. Placebo therapy (isotonic saline) was performed to patients with PD via single intranasal or intravenous injection. Effectiveness of the therapy was evaluated seven days post-transplantation according to the dynamics of motor symptoms. The severity of motor symptoms was evaluated on the basis of Section III of the Unified Parkinson’s Disease Rating Scale.
Statistical data processing was performed using the Statistica 8.0 package, programming language “Python” and Scipy 1.5 library). The data obtained are presented as the median with an interquartile interval (25–75th percentile). Comparison of the results of the two groups was carried out using nonparametric Wilcoxon criteria and the Mann – Whitney U test.
Results: The study included 34 patients (m:f – 22:12) with a diagnosis of PD. The average age of the patients was 56.0(47.0;64.0) years, the duration of the disease was 6.0(4.5;7.0) years, the severity of the disease according to the Hoehn & Yahr scale was 2.0(2.0;2.5) points.
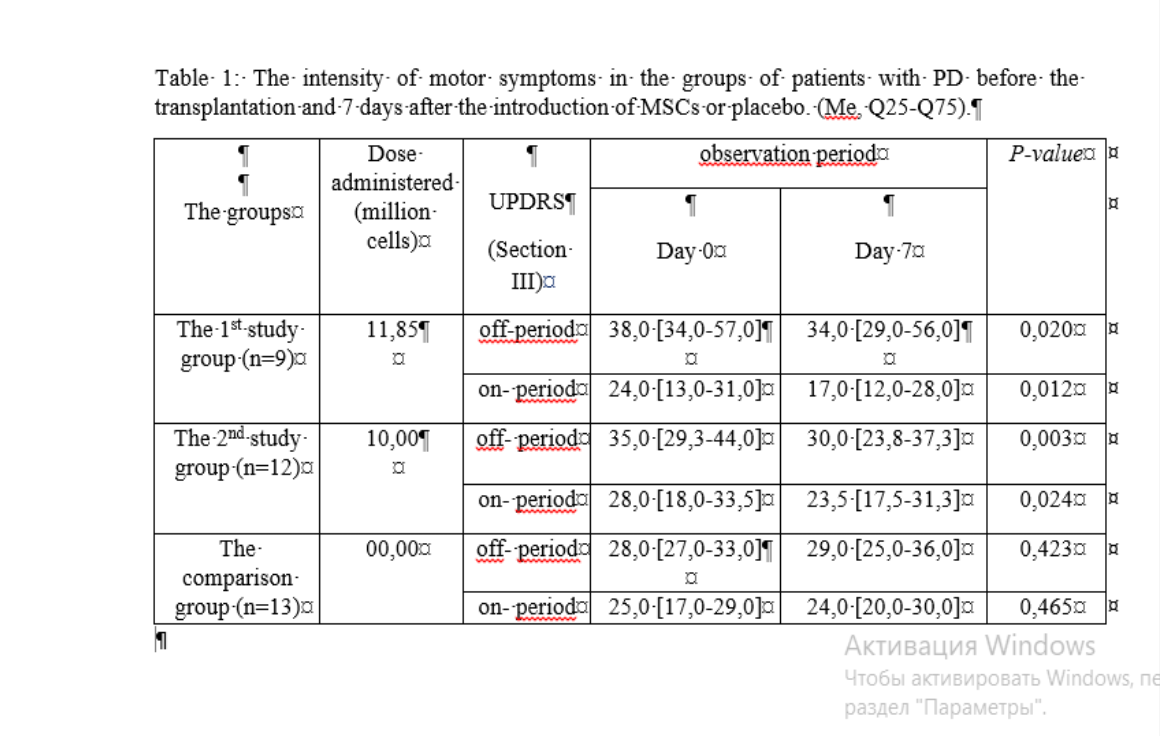
The 1st study group consisted of 9 patients with a diagnosis of PD, who received MSCs single intravenous injection, the average dose was Me=11.85[10.00-19.20]×106cells in 10.0 ml of the prepared solution. The 2nd study group consisted of 12 patients with PD, who received MSCs single intranasal injection, the average dose was Me=10,15[10,00-13,02]×106 cells in 5.0 ml of the prepared solution. The comparison group included 13 patients with PD that received placebo. The severity of motor symptoms in the early post-transplant period decreased in contrast to the comparison group [Table 1]. Moreover, the results of the 1st study group, where the patients received the low intravenous dose of MSCs, were similar to those of the 2nd study group, where the dose was standart for the intranasal administration.
Conclusion: The obtained data indicate the effectiveness of a small dose of intravenously administered MSCs that can be important for the improvement of the scheme and frequency of administration MSCs in PD.
To cite this abstract in AMA style:
V. Chyzhyk, V. Ponomarev, N. Aleinikava, A. Boika, M. Zafranskaya, D. Nizheharodava. The early post-transplant period in Parkinson’s disease: the low intravenous dose effect. [abstract]. Mov Disord. 2021; 36 (suppl 1). https://www.mdsabstracts.org/abstract/the-early-post-transplant-period-in-parkinsons-disease-the-low-intravenous-dose-effect/. Accessed July 5, 2025.« Back to MDS Virtual Congress 2021
MDS Abstracts - https://www.mdsabstracts.org/abstract/the-early-post-transplant-period-in-parkinsons-disease-the-low-intravenous-dose-effect/