Session Information
Date: Saturday, October 6, 2018
Session Title: Parkinson’s Disease: Clinical Trials, Pharmacology And Treatment
Session Time: 1:45pm-3:15pm
Location: Hall 3FG
Objective: To evaluate the effect of vocal fold injection augmentation with a temporary filler (carboxymethylcellulose gel) on voice and speech in Parkinson Disease (PD) patients with hypophonia and vocal fold atrophy.
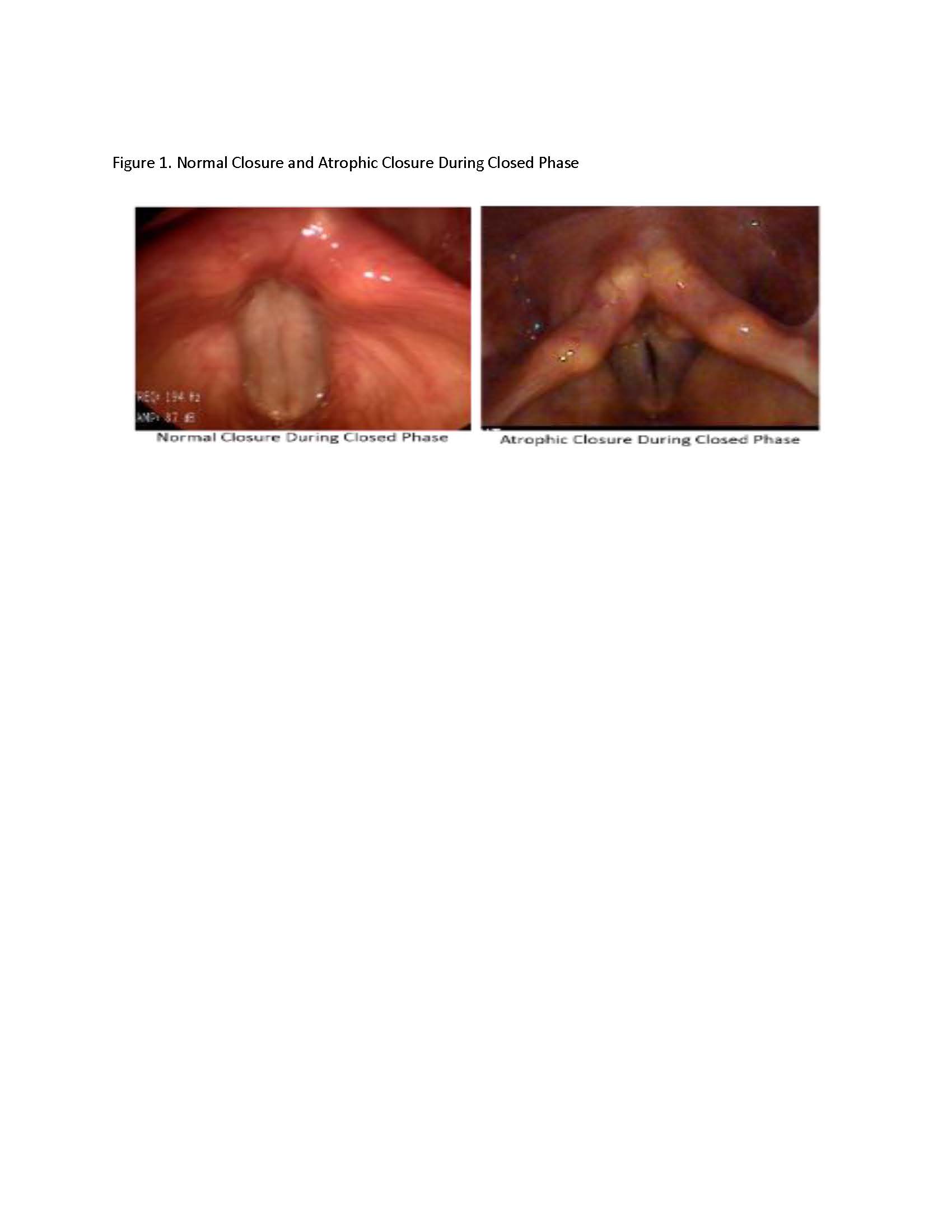
Background: Voice and speech impairments are extremely disabling problems affecting a majority of patients with PD. The etiology is multi factorial. Vocal fold atrophy causes glottic insufficiency, contributing to hypophonia. [Figure1] Vocal fold augmentation is a safe and effective treatment for vocal fold atrophy, but objective data in PD population are lacking.
Methods: PD patients with hypophonia and glottic insufficiency who underwent a vocal fold injection augmentation procedure were examined one month before, on the day of, and one month after injection. De-identified videostroboscopic exam and audio recordings of voice were evaluated by independent raters who were blinded to treatment state. The primary outcome measure was Consensus Auditory-Perceptual Evaluation of Voice (CAPE-V). Secondary outcome measures were glottic closure time, supraglottic constriction, binary assessment of voice, Eating Assessment Tool (EAT-10), and Voice Handicap Index-10 (VHI-10) questionnaires.
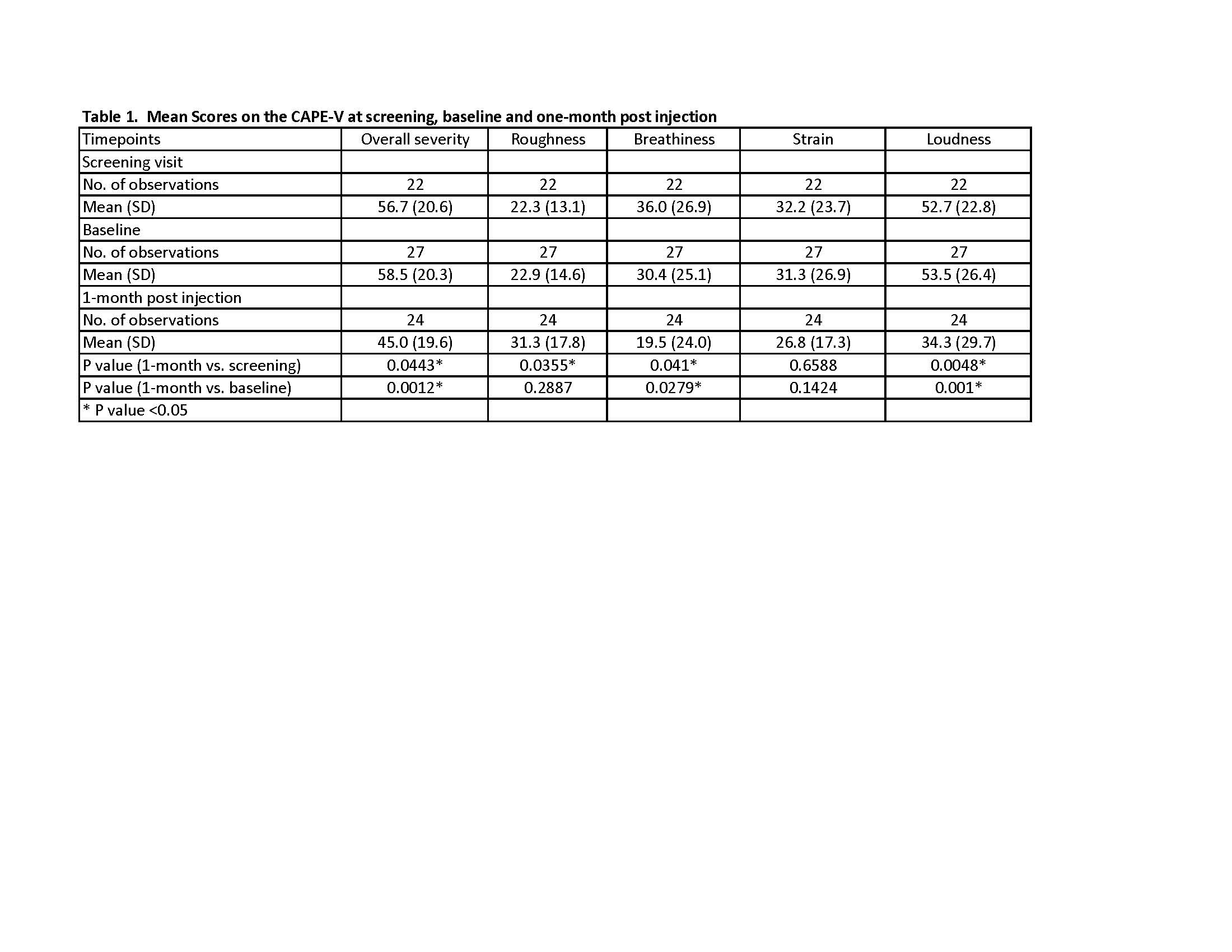
Results: 29 patients were enrolled, 4 females and 25 males, mean age 72.9 ± 5.9 years, mean disease duration 13.0 ± 10.6 years. Baseline means (SD) for CAPE-V sub scores were 58.5 ± 20.3 for overall severity, 22.9 ± 14.6 for roughness, 30.4 ± 25.1 for breathiness, 31.3 ± 26.9 for strain, and 53.5 ± 26.4 for loudness. After injection CAPE- V scores decreased (improved) for overall severity -11.4 ± 14.7, p=0.0012, breathiness -9.6 ± 19.5, p= 0.0279, and loudness -15.1 ± 19.1, p=0.001, [Table1, Figure2] with no significant change for other sub scores. Glottic closure improved significantly 10.8 ± 12.8, p=0.00024. Binary assessments estimated 68% probability of improvement of voice and 38% of supraglottic constriction. 25 (86%) patients reported subjective improvement, 24 (83%) requested for repeat injections. EAT-10 and VHI-10 changes were not significant. No adverse events were reported.
Conclusions: Our data demonstrated improvement in voice quality due to glottic closure in PD after vocal fold injection augmentation. For the first time the outcomes after the procedure in PD were analyzed in blinded fashion. Further studies of the effect of short- and long-acting vocal fold augmentation are warranted.
References: 1. Liotti M, Ramig L, Vogel D et al. Hypophonia in Parkinson’s disease: Neural correlates of voice treatment revealed by PET. Neurology. 2003;60(3):432-440. doi:10.1212/wnl.60.3.432. 2. Berke G, Gerratt B, Kreiman J, Jackson K. Treatment of Parkinson Hypophonia With Percutaneous Collagen Augmentation. Laryngoscope. 1999;109(8):1295-1299. doi:10.1097/00005537-199908000-00020.
To cite this abstract in AMA style:
O. Klepitskaya, J. Barr, Y. Liu, S. Sillau, J. Litts, D. Fink, M. Shaffer, J. Newcombe, A. Klepitskaya, M. Clary. The Impact of Vocal Fold Augmentation with Injection Laryngoplasty on Voice Quality in Patients with Parkinson Disease [abstract]. Mov Disord. 2018; 33 (suppl 2). https://www.mdsabstracts.org/abstract/the-impact-of-vocal-fold-augmentation-with-injection-laryngoplasty-on-voice-quality-in-patients-with-parkinson-disease/. Accessed May 14, 2024.« Back to 2018 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/the-impact-of-vocal-fold-augmentation-with-injection-laryngoplasty-on-voice-quality-in-patients-with-parkinson-disease/