Category: Dystonia: Clinical Trials and Therapy
Objective: To determine benefit from using methylphenidate for patients with uncontrolled blepharospasm and/or oromandibular dystonia.
Background: The preferred treatment for blepharospasm and related craniofacial dystonias involves local injections with botulinum toxins (1). Most treated individuals experience substantial benefits that are sustained for many years (2). However, satisfactory responses are challenging to obtain for some cases who either do not respond, have recurrent side effects, or have a short duration response. Additional therapies are therefore welcome.
Method: Consecutive subjects diagnosed with craniofacial dystonia with unsatisfactory response to BoNT-A injections, who tried methylphenidate as adjunctive treatment were prospectively recruited from the movement disorders clinic. Demographic information, duration of symptoms and subjective improvement measurements were obtained. Individual assessment videos were obtained via telehealth. Subjects were interviewed and asked to estimate the percentage of overall improvement. A one-minute video was recorded while at rest and looking directly towards the camera, before and 60-90-minutes after taking methylphenidate. Videos were reviewed in a randomized order by 4 physicians blinded to treatment status. Severity of craniofacial dystonia was assessed using available dystonia rating scales. Descriptive statistics are presented as average values, and changes before and after treatment were calculated in percentage of improvement.
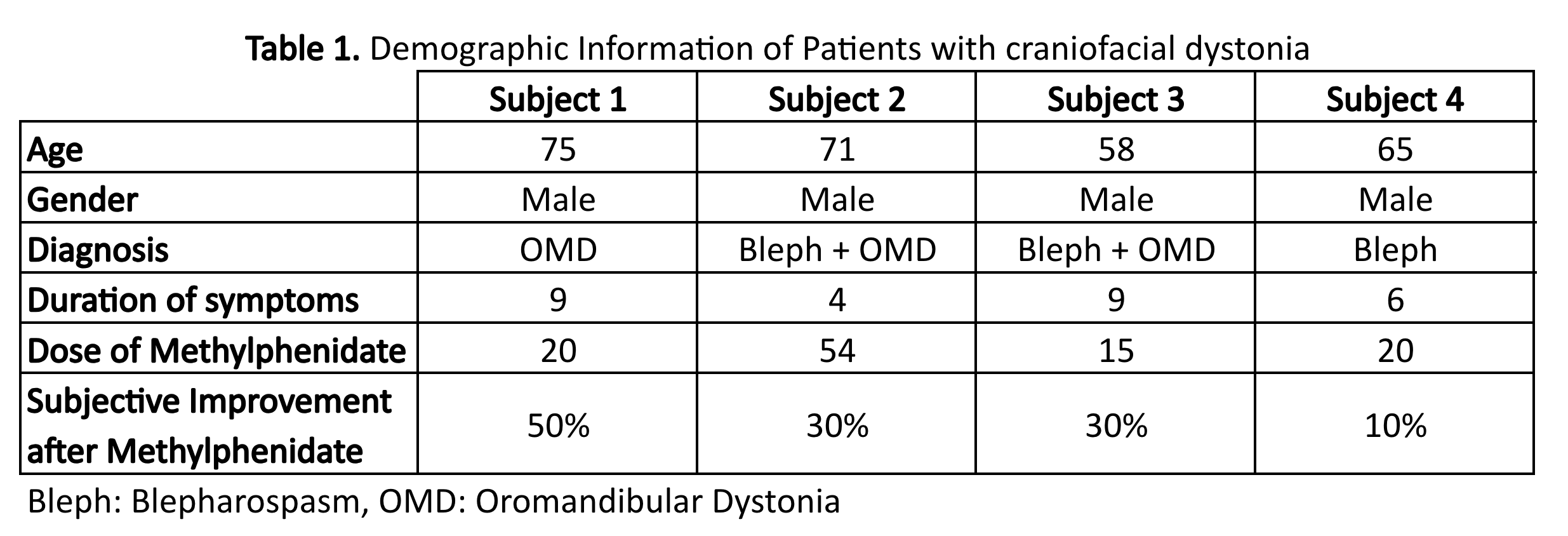
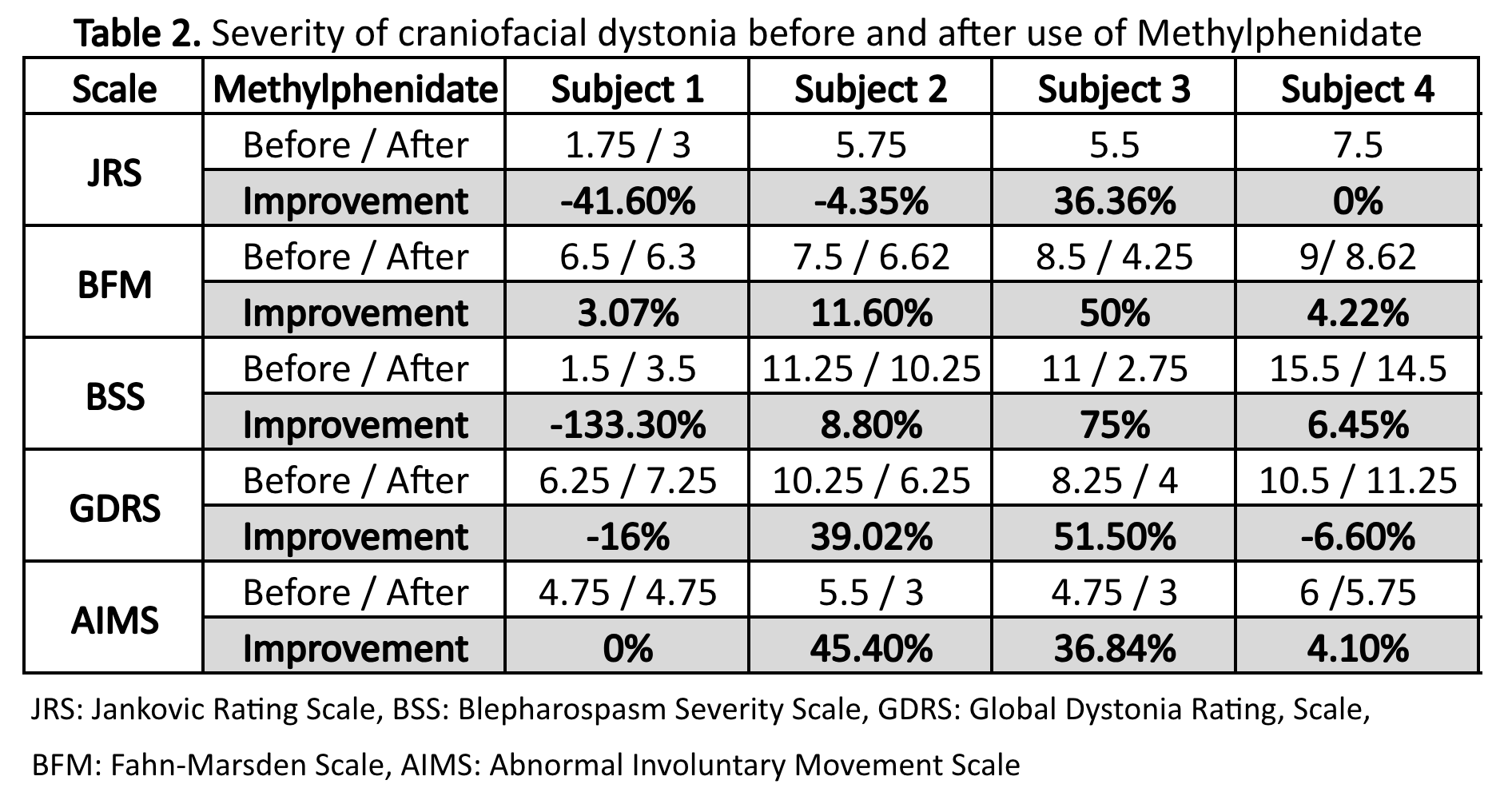
Results: Four male subjects with craniofacial dystonia were recruited, 1 with blepharospasm, one with OMD and 2 with blepharospasm and OMD, with a mean age of 67.2 years [58-75] and mean symptom duration of 7 years [4-9 years]. Average dose of Methylphenidate was 27.2 mg/day [10-54 mg/day]. Average patient subjective improvement after methylphenidate use was 28% [10-50%]. Objective improvement after taking Methylphenidate was present in all subjects, with more noticeable benefits in patients with blepharospasm and oromandibular dystonia (subjects 2 and 3). (Table 1) (Table 2)
Conclusion: Subjective and objective improvement was seen in all patients after taking Methylphenidate. A placebo effect after taking oral Methylphenidate is also possible. Prospective randomized placebo-control studies with a bigger sample size are needed to further support the use of Methylphenidate as an adjunctive treatment in focal dystonias.
Table 1
Table 2
References: 1. Price KM, Ramey NA, Richard MJ, Woodward DJ, Woodward JA. Can methylphenidate objectively provide relief in patients with uncontrolled blepharospasm? A pilot study using surface electromyography. Ophthalmic Plast Reconstr Surg. 2010 Sep-Oct;26(5):353-6. doi: 10.1097/IOP.0b013e3181cffa14. PMID: 20683274.
2. Eftekhari K, Choe CH, Vagefi MR, Gausas RE, Eckstein LA. Oral methylphenidate for the treatment of refractory facial dystonias. Ophthalmic Plast Reconstr Surg. 2015 May-Jun;31(3):e65-6. doi: 10.1097/IOP.0000000000000079. PMID: 25951177.
To cite this abstract in AMA style:
R. Lopez-Castellanos, H. Jinnah, M. Thayani. Treatment of Craniofacial Dystonias with Methylphenidate: A Case Series. [abstract]. Mov Disord. 2024; 39 (suppl 1). https://www.mdsabstracts.org/abstract/treatment-of-craniofacial-dystonias-with-methylphenidate-a-case-series/. Accessed July 13, 2025.« Back to 2024 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/treatment-of-craniofacial-dystonias-with-methylphenidate-a-case-series/