Session Information
Date: Sunday, October 7, 2018
Session Title: Phenomenology and Clinical Assessment Of Movement Disorders
Session Time: 1:45pm-3:15pm
Location: Hall 3FG
Objective: To evaluate the possibility of using remotely collected data to allocate newly diagnosed PD patients to phenotypic subgroups, to identify patients suitable for inclusion in neuroprotective clinical trials.
Background: Newer trials for neuroprotection are enriching study cohorts with those predicted to have more rapid progression. A recent publication has identified 5 subgroups in newly diagnosed PD, which may have prognostic significance, characterised by: (1) mild motor and non-motor disease; (2) poor posture and cognition; (3) severe tremor; (4) poor psychological well-being, RBD and sleep; and (5) severe motor and non-motor disease with poor well-being [1]. We evaluated whether it is possible to use remotely collected data from a wearable device combined with Non Motor Symptoms Questionnaire [2] (NMS Quest) data to allocate patients into relevant PD subgroups.
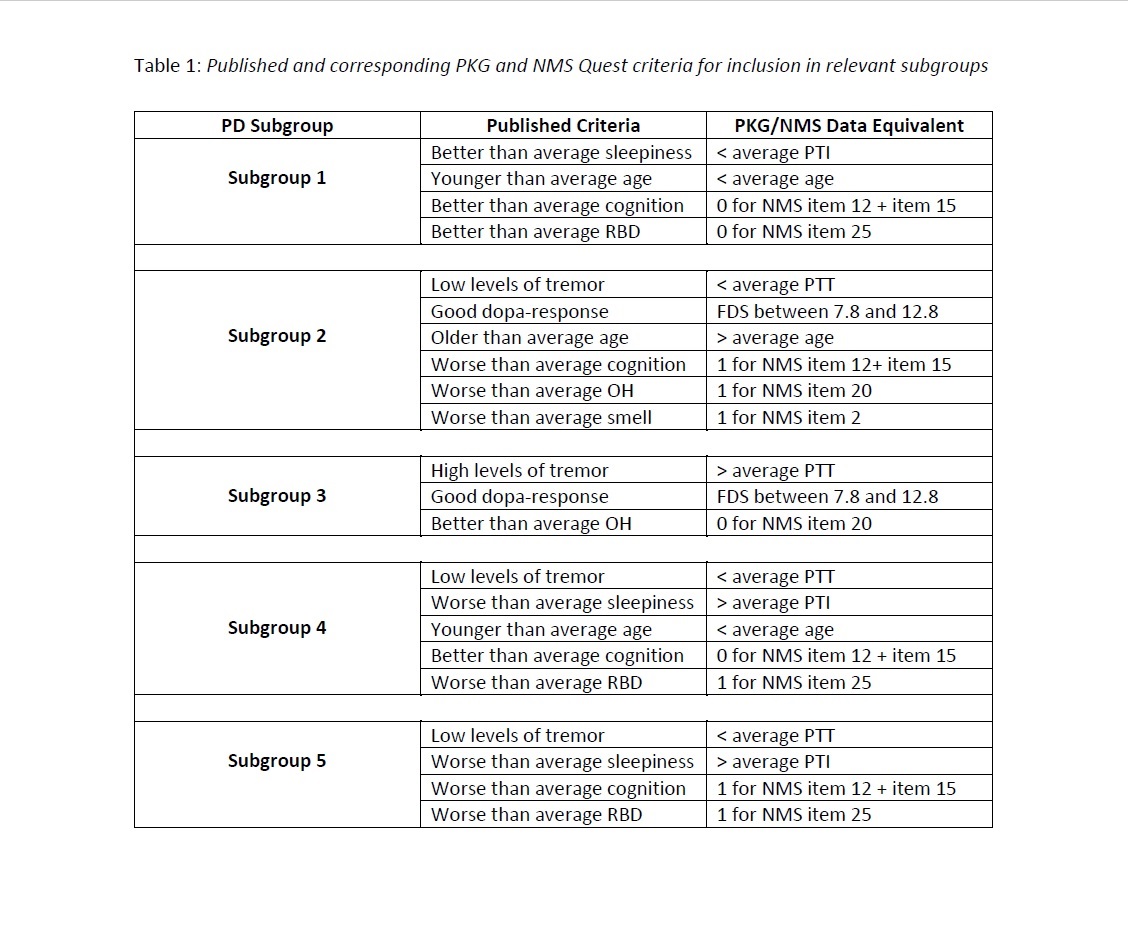
Methods: The Parkinson’s Kinetigraph (PKG™) is a wrist worn device developed for remote monitoring of symptoms in PD. PKG data and NMS Quest data were available for 62 newly diagnosed patients, including demographic information, a tremor score (PTT), an immobility score (PTI) (which correlates with daytime sleepiness) and a fluctuation and dyskinesia score (FDS) (which provides information on dopa-responsiveness). Patients were allocated to 1 of 5 subgroups based on a subset of corresponding PKG and NMS Quest data that specified criteria for inclusion in that subgroup. The relevant criteria for each subgroup are outlined in Table 1.
Results: 19% of patients met full criteria for inclusion in one of the five subgroups. 5% were allocated to Subgroup 1, 2% were allocated to Subgroup 2, 10% were allocated to Subgroup 3 and 2% were allocated to Subgroup 4. No patients met criteria for Subgroup 5. Out of the remaining patients, 51% failed to be allocated to a subgroup due to missing 1 of the required criteria for inclusion.
Conclusions: Preliminary analysis of our newly diagnosed patient cohort demonstrates it is possible to use remotely collected data to allocate patients to distinct phenotypic subgroups. This will be both clinically useful and valuable in identifying patients for trial inclusion. This is of particular value in clinical services where face-to-face clinic contact is limited.
References: 1. Lawton, M. et al. Parkinson’s Disease Subtypes in the Oxford Parkinson Disease Centre (OPDC) Discovery Cohort. J. Parkinsons. Dis. 5, 269–79 (2015). 2. Chaudhuri KR, Martinez‐Martin P, Schapira AH, Stocchi F, Sethi K, Odin P, Brown RG, Koller W, Barone P, MacPhee G, Kelly L. International multicenter pilot study of the first comprehensive self‐completed nonmotor symptoms questionnaire for Parkinson’s disease: the NMSQuest study. Movement Disorders. 2006 Jul 1;21(7):916-23.
To cite this abstract in AMA style:
T. Dominey, C. Carroll. Using remotely collected data to identify Parkinson’s disease (PD) subtypes [abstract]. Mov Disord. 2018; 33 (suppl 2). https://www.mdsabstracts.org/abstract/using-remotely-collected-data-to-identify-parkinsons-disease-pd-subtypes/. Accessed July 15, 2025.« Back to 2018 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/using-remotely-collected-data-to-identify-parkinsons-disease-pd-subtypes/