Session Information
Date: Wednesday, September 25, 2019
Session Title: Neuroimaging
Session Time: 1:15pm-2:45pm
Location: Les Muses Terrace, Level 3
Objective: To investigate spatial patterns and temporal evolution of substantia nigra (SN) iron accumulation in Parkinson’s disease (PD) using quantitative susceptibility mapping (QSM), an indirect marker of iron content.
Background: PD is characterized in part by the progressive accumulation of iron within the SN [1] however, its spatial and temporal dynamics remain relatively poorly understood. Previous studies using diffusion tensor imaging SN assessments in PD have shown the more caudal SN, mostly inferior to the red nucleus (RN), to be the most sensitive location to detect both cross-sectional and longitudinal changes [2, 3]. However, rostral/caudal differences in SN iron in PD have not been extensively examined.
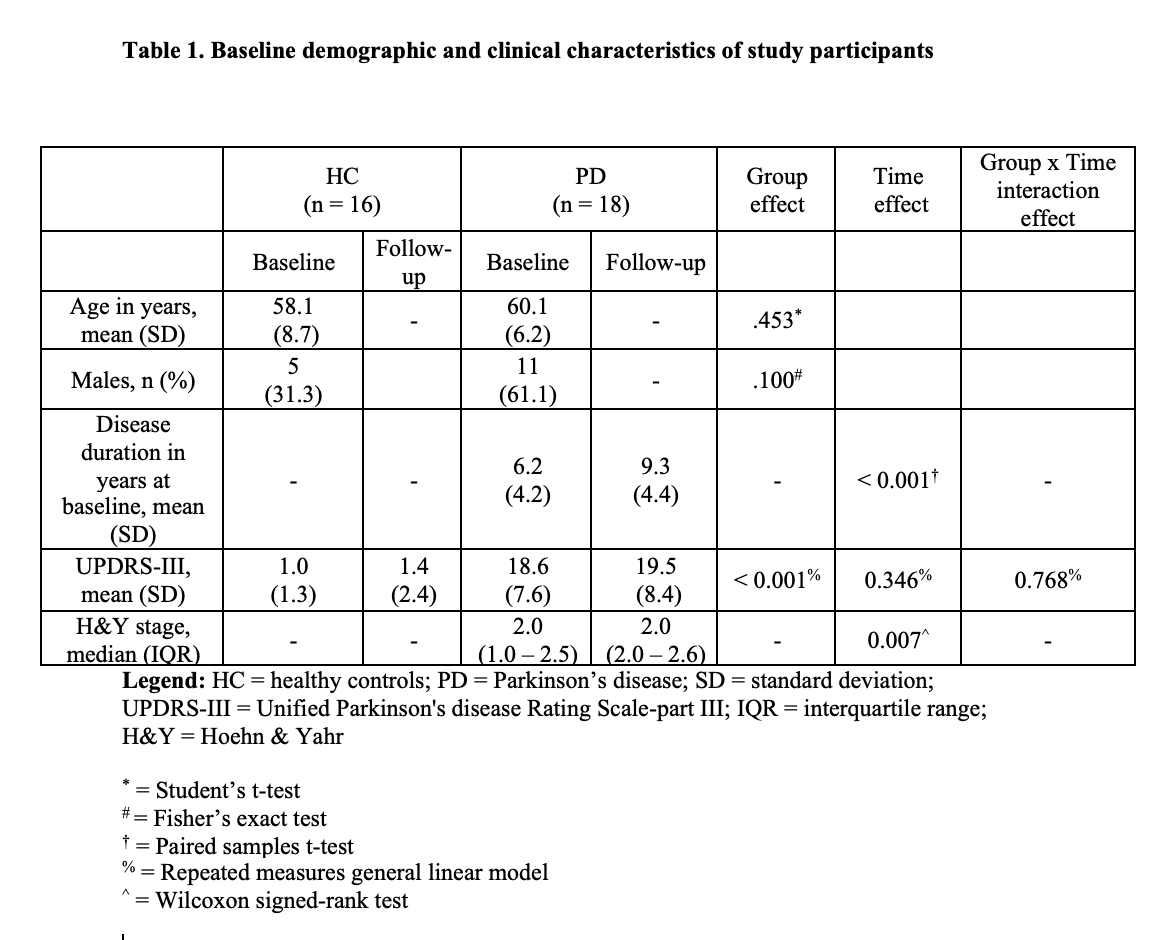
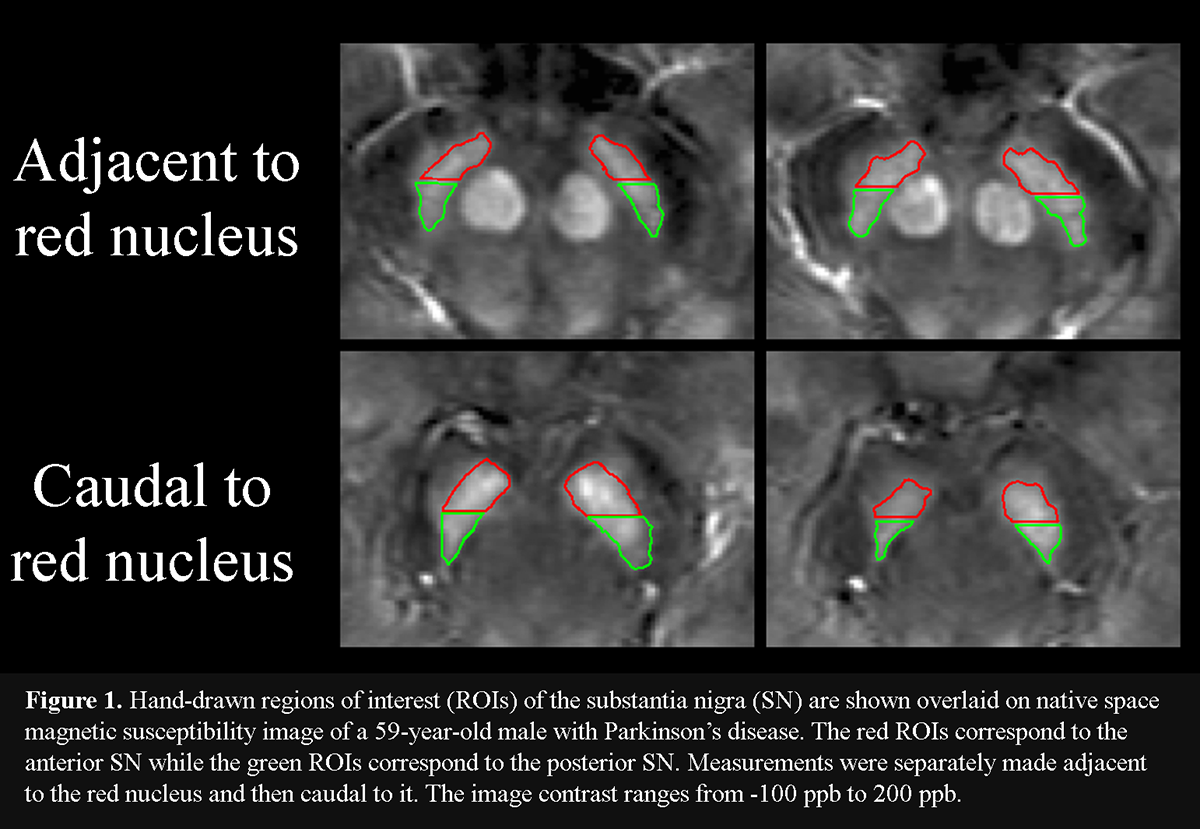
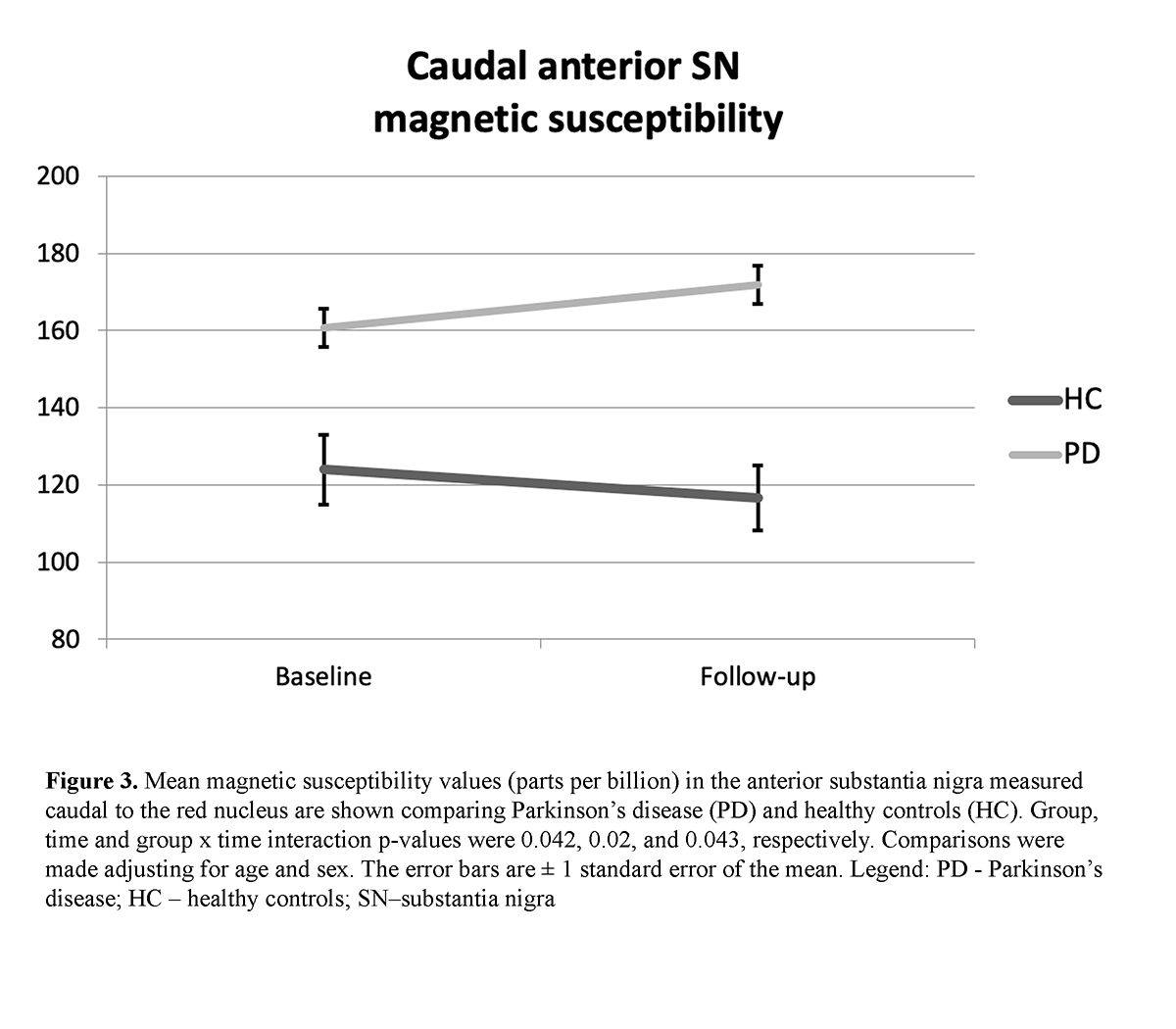
Method: Eighteen PD patients (mean disease duration = 7.1 years), receiving dopaminergic therapy, and 16 healthy controls (HC) were scanned with 3T MRI at baseline and 3 years later using QSM [table1]. Iron was assessed separately in the posterior (pSN) and anterior (aSN) at rostral and caudal levels of the SN. Two consecutive slices were selected rostrally, where the RN was most prominently visible, defined as the portion of the SN “adjacent to the RN”. Next, two consecutive slices were chosen just caudal to where the RN was either barely or no longer visible, defined as the portion of the SN “caudal to the RN”. pSN and aSN regions were defined in both rostral and caudal levels by splitting the original SN ROI at the midway point along the anterior-posterior axis [figure1]. ROIs were drawn blinded to clinical, demographic and time point information. P-values were corrected for the false discovery rate.
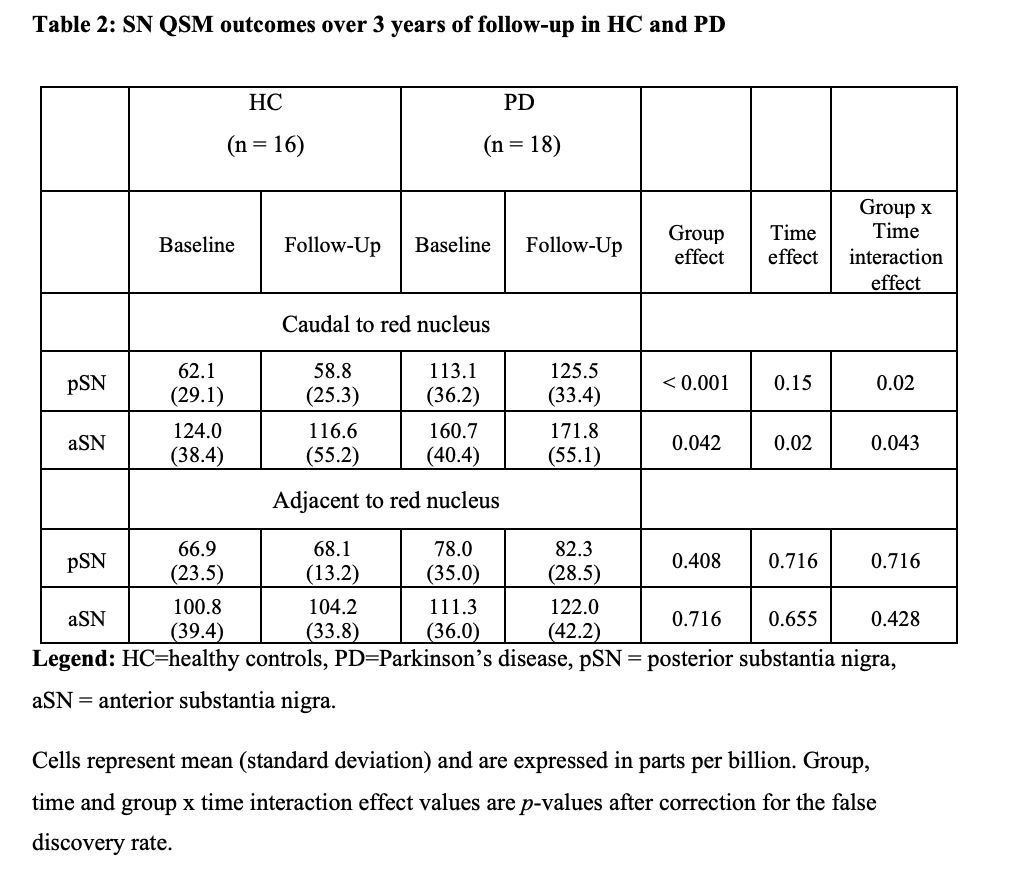
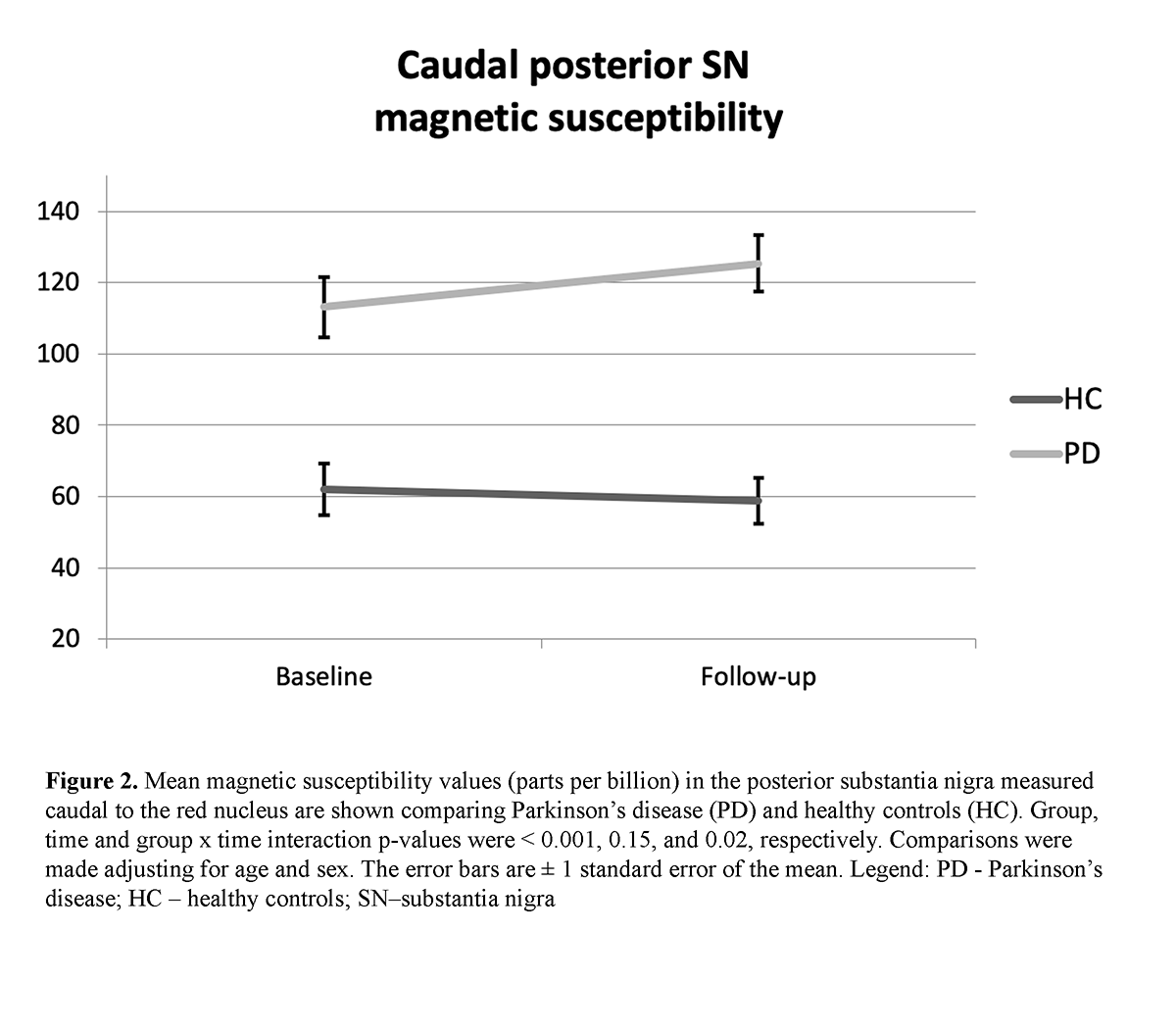
Results: A significant group effect was found for the caudal pSN (p < 0.001) and aSN (p = 0.042) QSM as well as significant group x time interaction effects (p = 0.02 and p = 0.043, respectively [table2] [figure2] [figure3]. A significant intra-group change over 3 years of follow-up was found only in the caudal pSN of PD (p = 0.012) but not HC. No significant effects were detected for any rostral SN measures. No associations were identified with clinical measures.
Conclusion: We found both cross-sectional and longitudinal SN iron changes to be confined to its more caudal location in PD. Because pathology studies also show the caudal SN to degenerate early and to the greatest extent in PD [4], assessment of iron levels by QSM in this area may potentially represent a disease progression biomarker in PD.
References: 1. Sofic E, Paulus W, Jellinger K, Riederer P, Youdim MB. Selective increase of iron in substantia nigra zona compacta of parkinsonian brains. J Neurochem. 1991;56(3):978-82. 2. Ofori E, Krismer F, Burciu RG, Pasternak O, McCracken JL, Lewis MM, et al. Free water improves detection of changes in the substantia nigra in parkinsonism: A multisite study. Mov Disord. 2017;32(10):1457-64. 3. Ofori E, Pasternak O, Planetta PJ, Li H, Burciu RG, Snyder AF, et al. Longitudinal changes in free-water within the substantia nigra of Parkinson’s disease. Brain. 2015;138(Pt 8):2322-31. 4. Damier P, Hirsch EC, Agid Y, Graybiel AM. The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson’s disease. Brain. 1999;122 ( Pt 8):1437-48.
To cite this abstract in AMA style:
N. Bergsland, R. Zivadinov, F. Schweser, J. Hagemeier, D. Lichter, T. Guttuso, JR.. Caudal posterior substantia nigra iron increases longitudinally in Parkinson’s disease [abstract]. Mov Disord. 2019; 34 (suppl 2). https://www.mdsabstracts.org/abstract/caudal-posterior-substantia-nigra-iron-increases-longitudinally-in-parkinsons-disease/. Accessed February 27, 2026.« Back to 2019 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/caudal-posterior-substantia-nigra-iron-increases-longitudinally-in-parkinsons-disease/