Session Information
Date: Thursday, June 8, 2017
Session Title: Parkinson’s Disease: Clinical Trials, Pharmacology And Treatment
Session Time: 1:15pm-2:45pm
Location: Exhibit Hall C
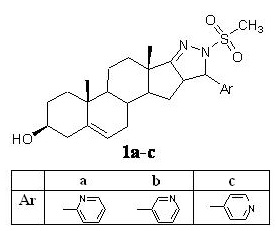
Objective: This present study is mainly focused at design and synthesis of new therapeutically useful steroidal neuroprotective agents against neuroinflammation which involved in the progression of neurodegenerative disorders such as Alzheimer’s Disease, Parkinson’s Disease, and Multiple Sclerosis. The new androstane derivative has been synthesized by fusing N-methylsulfonyl substituted pyridylpyrazoline moieties at [17,16-c] position of the steroid. These D ring modified heterosteroids were then explored for their antiparkinson and antioxidant effect.
Background:
Parkinson’s Disease (PD) is a progressive, disabling neurodegenerative disorder characterized by an insidious onset with variable expression of motor, vegetative, sensory and psychopathological symptoms. Recent literature reports indicated that neuroinflammation cause dopaminergic neuron death that involved in progression of PD.
Methods:
Aldol condensation of dehydroepiandrosterone with requisite pyridine carboxaldehyde in basic medium gave corresponding 16-arylidene steroidal derivatives, treatment of which with hydrazine hydrate in refluxing methanol afforded pyrazoline substituted steroids. Pyrazoline intermediates were further treated with mesyl chloride at 0° C to afford target N-methyl sulfonyl substituted pyrazoline steroids 1a-c. Rats (male wistar) were anesthetized with thiopental sodium (45 mg/kg, i.p.), stereotaxic surgery has been done and intranigral injection of LPS (10µg in 2µl) was infused into left substantia nigra using the Hamilton microsyringe.3 Heterosteroids were evaluated against behavioral alternations using actophotometer, elevated plus maze at dose 2mg/kg after 7th, 14th and 21st day of LPS administration. Biochemical estimation of different makers for neuroinflammation, cholinergic activity and oxidative stress has also been carried out.
Results:
The synthetic heterosteroids were characterized using IR, 1H NMR. All heterosteroids 1a-c exhibited potent activity against PD. The pyridin-4-yl group substituted steroid 1c displayed activity comparable to that of standards dexamethasone and celecoxib.
Conclusions:
The findings suggests that N-methanesulfonylpyrazolinyl substituted exhibit potent anti-neuroinflammatory activity and could be useful for the prevention of PD and oxidative stress.
References: 1. Herrera EJ, Viano JC, Caceres M, Costello G, Suarez M, Suarez JC. Posteroventral pallidotomy in Parkinson’s disease. Acta Neurochir. 2000, 142, 169–175. 1. Tansey MG, Goldberg MS. Neuroinflammation in Parkinson’s disease: its role in neuronal death and implications for therapeutic intervention. Neurobiology of disease. 2010, 37, 510-518
To cite this abstract in AMA style:
R. Singh. Antiparkinson and antioxidant effect of N-Methanesulfonylpyrazolinyl substituted Heterosteroids in LPS induced Neuroinflammation Model of Rats [abstract]. Mov Disord. 2017; 32 (suppl 2). https://www.mdsabstracts.org/abstract/antiparkinson-and-antioxidant-effect-of-n-methanesulfonylpyrazolinyl-substituted-heterosteroids-in-lps-induced-neuroinflammation-model-of-rats/. Accessed July 18, 2025.« Back to 2017 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/antiparkinson-and-antioxidant-effect-of-n-methanesulfonylpyrazolinyl-substituted-heterosteroids-in-lps-induced-neuroinflammation-model-of-rats/