Session Information
Date: Monday, September 23, 2019
Session Title: Genetics
Session Time: 1:45pm-3:15pm
Location: Les Muses Terrace, Level 3
Objective: We aimed to investigate clinical and pathological characteristics in hereditary diffuse leukoencephalopathy with spheroids (HDLS) patients and explore the potential impact of colony-stimulating factor 1 receptor gene (CSF1R) mutations.
Background: HDLS is a rare white-matter encephalopathy characterized by motor and neuropsychiatric symptoms due to CSF1R mutation. The pathogenesis still remains unknown.
Method: In order to investigate clinical and pathological characteristics in HDLS patients and explore the potential impact of CSF1R mutations, we analyzed clinical manifestations of 15 patients from 10 unrelated families and performed brain biopsy in 2 cases. Next generation sequencing was conducted for 10 probands to confirm the diagnosis. Sanger sequencing and segregation analysis were utilized to substantiate findings. Functional examination of identified mutations was further explored.
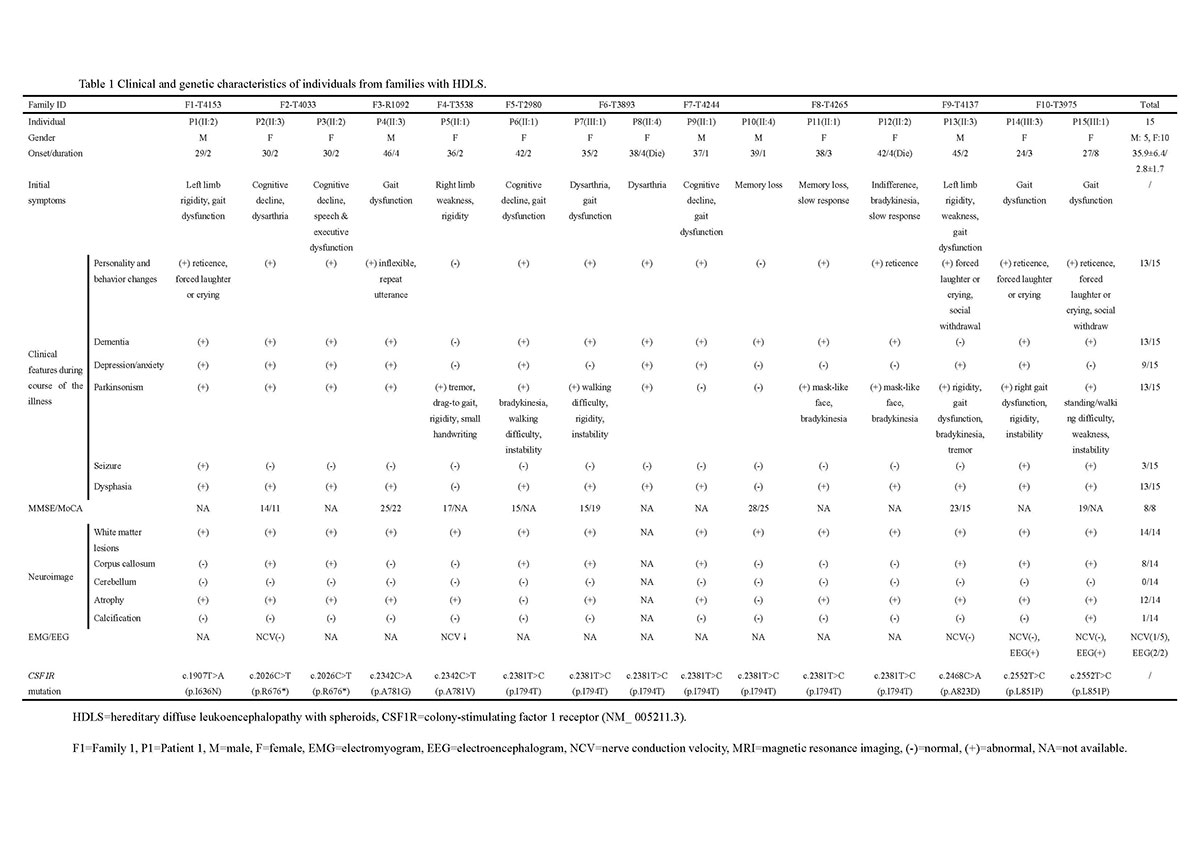
Results: Clinical and neuroimaging characteristics were summarized. The average age at onset was 35.9±6.4 years (range 24-46 years old). Younger age of onset was observed in female than male (34.2 vs. 39.2 years). The most common initial symptoms were speech dysfunction, cognitive decline and parkinsonism symptoms. One patient also had marked peripheral neuropathy. Brain biopsy of two cases showed typical pathological changes, including myelin loss, axonal spheroids, phosphorylated neurofilament and activated macrophages. Electron microscopy disclosed increased mitochondria and disorganized neurofilaments in ballooned axons. A total of 7 pathogenic variants (4 novel, 3 documented) were identified, which were further testified by functional study, showing autophosphorylation deficiency in all the mutants. The level of microtubule associated protein 1 light chain 3-II (LC3-II), a classical marker of autophagy, was significantly lower in mutants expressed cells than wild type group by western blotting and immunofluorescence staining.
Conclusion: This study suggests that polyneuropathy may be a part of CSF1R deficiency which deserves further study with longer follow-up and more patients enrolled. Our findings also support the haploinsufficiency hypothesis in pathogenesis and partial loss-of-autophosphorylation of CSF1R can lead to a relatively mild phenotype. Autophagy abnormality may play a role in the disease. Repairing or promoting the phosphorylation level of mutant CSF1R may shed light on therapeutic targets in the future.
References: 1. Axelsson R, Röyttä M, Sourander P, Akesson HO, Andersen O. Hereditary diffuse leucoencephalopathy with spheroids. Acta Psychiatr Scand Suppl 1984; 314:1-65. 2. Rademakers R, Baker M, Nicholson AM, et al. Mutations in the colony stimulating factor 1 receptor (CSF1R) gene cause hereditary diffuse leukoencephalopathy with spheroids. Nat Genet 2011;44(2):200-205. 3. Roytta, M. The First Neuropathological Studies on HDLS. J Neuropathol Exp Neurol 2015;74(6):587. 4. Marotti JD, Tobias S, Fratkin JD, Powers JM, Rhodes CH. Adult onset leukodystrophy with neuroaxonal spheroids and pigmented glia: report of a family, historical perspective, and review of the literature. Acta Neuropathol 2004;107(6):481-488. 5. Itoh K, Shiga K, Shimizu K, Muranishi M, Nakagawa M, Fushiki S. Autosomal dominant leukodystrophy with axonal spheroids and pigmented glia: clinical and neuropathological characteristics. Acta Neuropathol 2006;111(1):39-45. 6. Sundal C, Lash J, Aasly J, et al. Hereditary diffuse leukoencephalopathy with axonal spheroids (HDLS): a misdiagnosed disease entity. J Neurol Sci 2012; 314(1-2):130-137. 7. Konno T, Broderick DF, Mezaki N, et al. Diagnostic Value of Brain Calcifications in Adult-Onset Leukoencephalopathy with Axonal Spheroids and Pigmented Glia. AJNR Am J Neuroradiol 2017;38(1):77-83. 8. Kleinfeld K, Mobley B, Hedera P, Wegner A, Sriram S, Pawate S. Adult-onset leukoencephalopathy with neuroaxonal spheroids and pigmented glia: report of five cases and a new mutation. J Neurol 2013;260(2):558-571. 9. Konno T, Kasanuki K, Ikeuchi T, Dickson DW, Wszolek ZK. CSF1R-related leukoencephalopathy: A major player in primary microgliopathies. Neurology 2018;91(24):1092-1104. 10. Lynch DS, Zhang WJ, Lakshmanan R, et al. Analysis of Mutations in AARS2 in a Series of CSF1R-Negative Patients With Adult-Onset Leukoencephalopathy With Axonal Spheroids and Pigmented Glia. JAMA Neurol 2016;73(12):1433-1439. 11. Prinz M, Priller J. Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat Rev Neurosci 2014;15(5):300-312. 12. Luo J, Elwood F, Britschgi M, et al. Colony-stimulating factor 1 receptor (CSF1R) signaling in injured neurons facilitates protection and survival. J Exp Med 2013;210(1):157-172. 13. Oosterhof N, Kuil LE, van der Linde HC, et al. Colony-Stimulating Factor 1 Receptor (CSF1R) Regulates Microglia Density and Distribution, but Not Microglia Differentiation In Vivo. Cell Rep 2018;24(5):1203-1217. 14. Jacquel A, Obba S, Boyer L, et al. Autophagy is required for CSF-1-induced macrophagic differentiation and acquisition of phagocytic functions. Blood 2012;119(19):4527-4531. 15. Konno T, Miura T, Harriott AM, et al. Partial loss of function of colony-stimulating factor 1 receptor in a patient with white matter abnormalities. Eur J Neurol 2018;25(6):875-881. 16. Nicholson AM, Baker MC, Finch NA, et al. CSF1R mutations link POLD and HDLS as a single disease entity. Neurology 2013;80(11):1033-1040. 17. Konno T, Tada M, Tada M, et al. Haploinsufficiency of CSF-1R and clinicopathologic characterization in patients with HDLS. Neurology 2014; 82(2):139-148. 18. Eichler FS, Li J, Guo Y, et al. CSF1R mosaicism in a family with hereditary diffuse leukoencephalopathy with spheroids. Brain 2016;139(Pt 6):1666-1672. 19. Konno T, Yoshida K, Mizuta I, et al. Diagnostic criteria for adult-onset leukoencephalopathy with axonal spheroids and pigmented glia due to CSF1R mutation. Eur J Neurol 2018;25(1):142-147. 20. Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015;17(5):405-424. 21. Ayrignac X, Carra-Dalliere C, Menjot de Champfleur N, et al. Adult-onset genetic leukoencephalopathies: a MRI pattern-based approach in a comprehensive study of 154 patients. Brain 2015;138(Pt 2):284-292. 22. Obba S, Hizir Z, Boyer L, et al. The PRKAA1/AMPKalpha1 pathway triggers autophagy during CSF1-induced human monocyte differentiation and is a potential target in CMML. Autophagy 2015;11(7):1114-1129. 23. Sundal C, Baker M, Karrenbauer V, et al. Hereditary diffuse leukoencephalopathy with spheroids with phenotype of primary progressive multiple sclerosis. Eur J Neurol 2015;22(2):328-333. 24. Guerreiro R, Kara E, Le Ber I, et al. Genetic analysis of inherited leukodystrophies: genotype-phenotype correlations in the CSF1R gene. JAMA Neurol 2013;70(7):875-882. 25. Freeman SH, Hyman BT, Sims KB. Adult onset leukodystrophy with neuroaxonal spheroids: clinical, neuroimaging and neuropathologic observations. Brain Pathol 2009;19(1):39-47. 26. Di Donato I, Stabile C, Bianchi S, et al. A Novel CSF1R Mutation in a Patient with Clinical and Neuroradiological Features of Hereditary Diffuse Leukoencephalopathy with Axonal Spheroids. J Alzheimers Dis 2015;47(2):319-322. 27. Scheijen B, Griffin JD. Tyrosine kinase oncogenes in normal hematopoiesis and hematological disease. Oncogene 2002;21(21):3314-33. 28. Pridans C, Sauter KA, Baer K, Kissel H, Hume DA. CSF1R mutations in hereditary diffuse leukoencephalopathy with spheroids are loss of function. Sci Rep 2013;3:3013. 29. Stabile C, Taglia I, Battisti C, Bianchi S, Federico A. Hereditary diffuse leukoencephalopathy with axonal spheroids (HDLS): update on molecular genetics. Neurol Sci 2016;37(9):1565-1569. 30. Miura T, Mezaki N, Konno T, et al. Identification and functional characterization of novel mutations including frameshift mutation in exon 4 of CSF1R in patients with adult-onset leukoencephalopathy with axonal spheroids and pigmented glia. J Neurol 2018;265(10):2415-2424. 31. Pottier C, Bieniek KF, Finch N, et al. Whole-genome sequencing reveals important role for TBK1 and OPTN mutations in frontotemporal lobar degeneration without motor neuron disease. Acta Neuropathol 2015;130(1):77-92. 32. Klionsky DJ, Abdelmohsen K, Abe A, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 2016;12(1):1-222. 33. Percin GI, Eitler J, Kranz A, Fu J, Pollard JW, Naumann R, Waskow C. CSF1R regulates the dendritic cell pool size in adult mice via embryo-derived tissue-resident macrophages. Nat Commun 2018;9(1):5279. 34. Su P, Zhang J, Wang D, Zhao F, Cao Z, Aschner M, Luo W. The role of autophagy in modulation of neuroinflammation in microglia. Neuroscience 2016;319:155-167.
To cite this abstract in AMA style:
W. Tian, L. Cao, S. Chen. Clinicopathologic Characterization and Abnormal Autophagy of HDLS [abstract]. Mov Disord. 2019; 34 (suppl 2). https://www.mdsabstracts.org/abstract/clinicopathologic-characterization-and-abnormal-autophagy-of-hdls/. Accessed July 12, 2025.« Back to 2019 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/clinicopathologic-characterization-and-abnormal-autophagy-of-hdls/