Category: Surgical Therapy: Parkinson's Disease
Objective: We conducted a critical appraisal of the literature on pregnant patients using deep brain stimulation (DBS) in order to assess safety outcomes for maternal and fetal health and identify clinical changes throughout this period.
Background: DBS holds promise for significantly improving the lives of those with neurological and psychiatric conditions. Despite its positive impact on many patients, pregnant women have faced restrictions due to safety concerns, leading to their exclusion from clinical trials. This creates a notable gap in access to treatment for individuals who could otherwise benefit from this procedure.
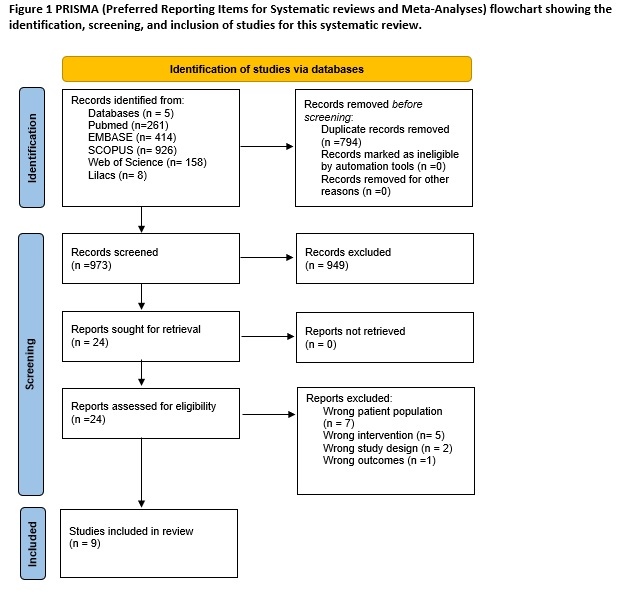
Method: A systematic search was conducted according to PRISMA guidelines [figure1] in MEDLINE, EMBASE, Web of Science, Scopus, and Lilacs for studies published from inception to February 25, 2024. Studies evaluating pregnant patients with active DBS systems implanted before their pregnancy were included. No age or language restrictions were imposed. Studies that used secondary data, had DBS discontinuation, or did not follow the pregnancy through delivery were excluded. Quality was assessed using the NewCastle-Ottawa scale for observational studies and its adapted version for case reports and case series.
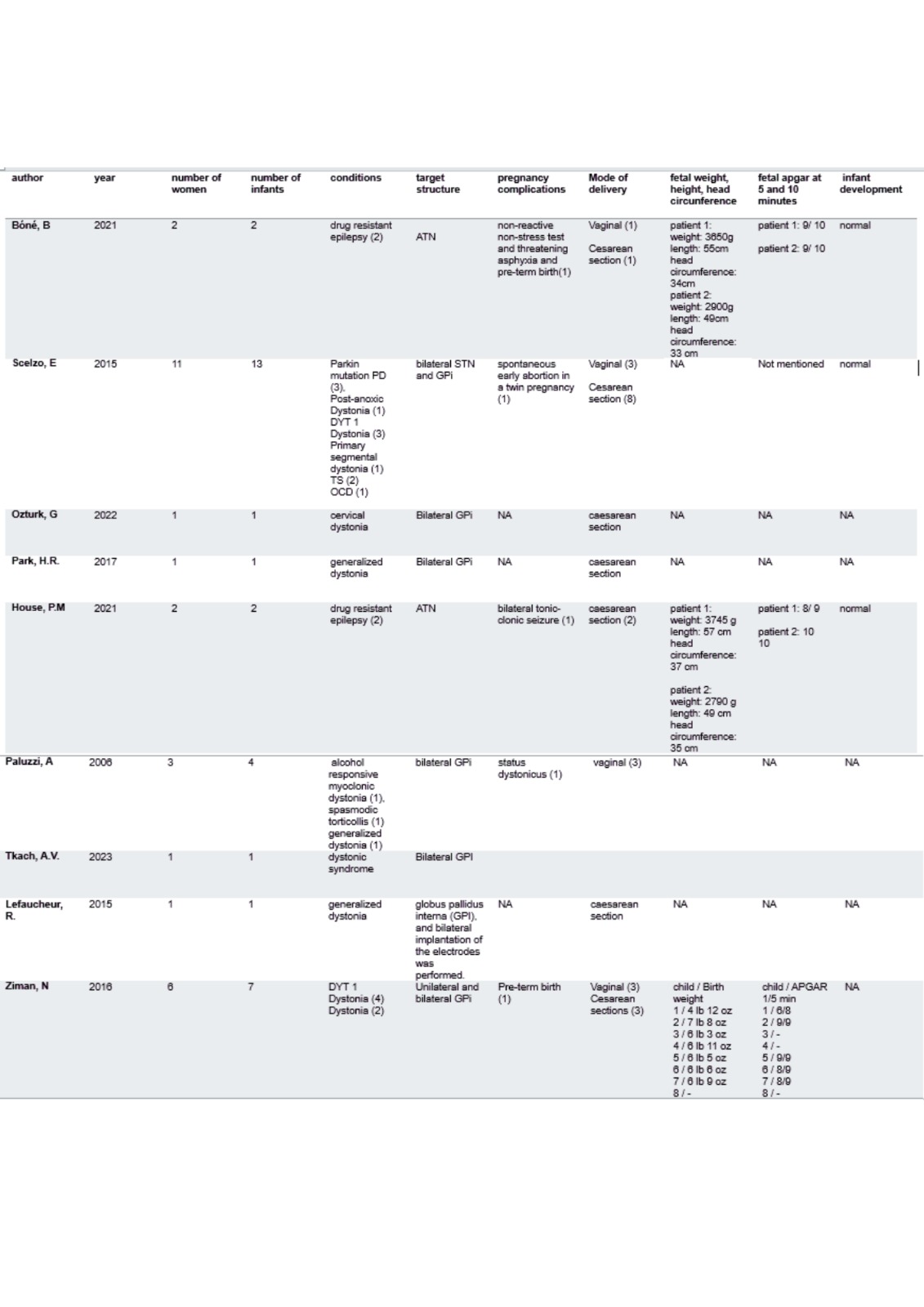
Results: We screened 973 studies and selected 10. A total of 28 women and 32 infants were assessed [table1]. Age of implantation ranged from 11-33 years old. Conditions treated include drug resistant epilepsy (n=4), dystonia (n=18), obsessive compulsive disorder (n=1), parkinson disease (n=3) and Tourette’s syndrome (n=2). Most patients did not experience worsening of the disease during pregnancy although 4 women presented transient deterioration that was solved at delivery. Complications during pregnancy include tonic-clonic seizure (n=1), spontaneous abortion (n=1) and status dystonicus (n=1). Preterm deliveries were reported in 2 patients. Follow up ranged from 7 months to 9 years. No malformations were reported and neurodevelopment was normal in all followed infants. Quality assessment showed medium to high risk of bias.
Conclusion: Our findings suggest that the use of DBS during pregnancy is safe and effective. Complications for pregnant women and her fetuses were infrequent and transient and development of infants was normal in all cases. Although our results are encouraging, randomized trials are needed to confirm these findings.
Figure 1
Table 1
To cite this abstract in AMA style:
N. Pacheco-Barrios, K. Acurio, F. Váscones-Román, A. Flores-Gavino, I. Calisaya-Madriaga, W. Rios-Garcia, L. Aguilar-Alvarez, J. Rolston. Deep Brain Stimulation during pregnancy: A systematic review of the literature [abstract]. Mov Disord. 2024; 39 (suppl 1). https://www.mdsabstracts.org/abstract/deep-brain-stimulation-during-pregnancy-a-systematic-review-of-the-literature/. Accessed July 1, 2025.« Back to 2024 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/deep-brain-stimulation-during-pregnancy-a-systematic-review-of-the-literature/