Category: Parkinsonism, Atypical: MSA
Objective: We present two patients initially diagnosed as familial MSA until one of the patients was proven to be metachromatic leukodystrophy (MLD).
Background: Multiple system atrophy (MSA) is generally a sporadic disease and its diagnostic criteria include a negative family history as an essential feature. [1-2] However, there have been reports of familial MSA cases and some were pathologically-proven. [3-4]
Method: Retrospective chart review was done for 2 cases. (SNUH4, SNUH5)
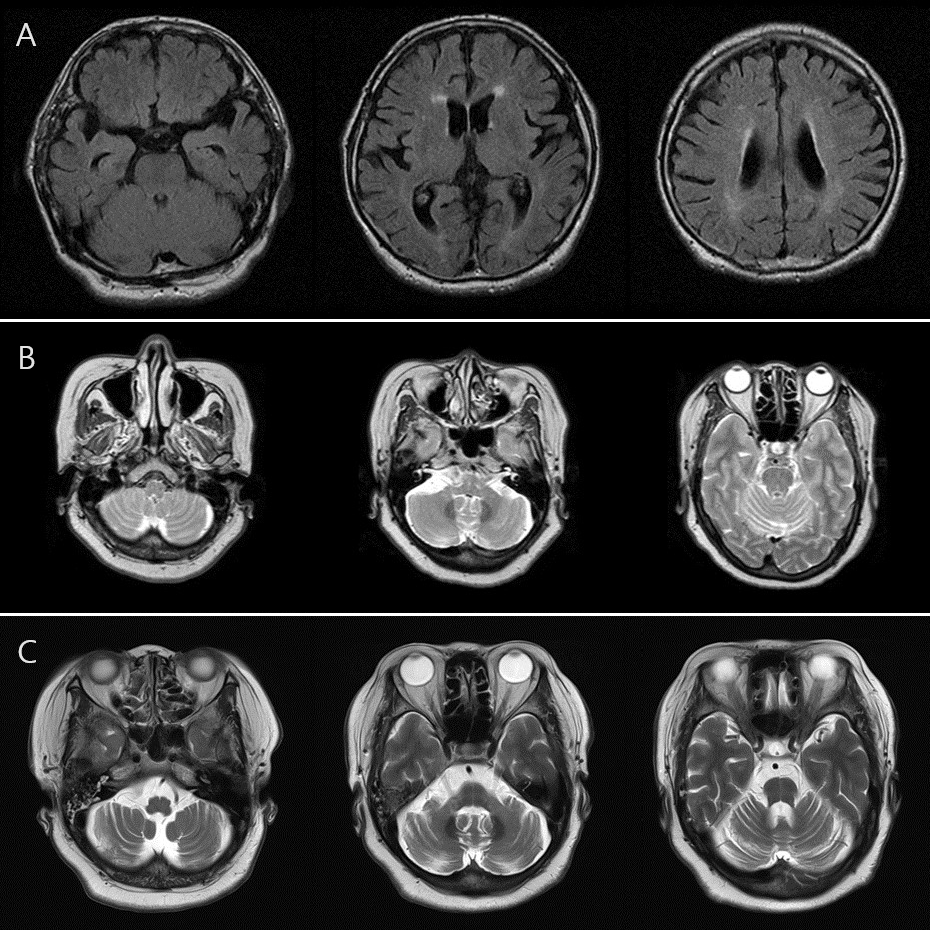
Results: A 77-year-old male (patient SNUH4) was diagnosed as MSA-P with progressive autonomic dysfunction, postural instability, bradykinesia, and poor L-dopa responsiveness. A 55-year-old female (patient SNUH5) was diagnosed as MSA-C with severe postural instability, autonomic dysfunction, ataxia, and cerebellar and pontine atrophy on brain MRI. [figure1, SNUH4’s brain MRI T2 FLAIR axial images showing mild periventricular small vessel disease. (age 77) (B) SNUH5’s brain MRI T2 axial images showing mild diffuse cerebellar atrophy. (age 52) (C) SNUH5’s follow-up images showing progressed cerebellar atrophy and pontine atrophy (age 55).]
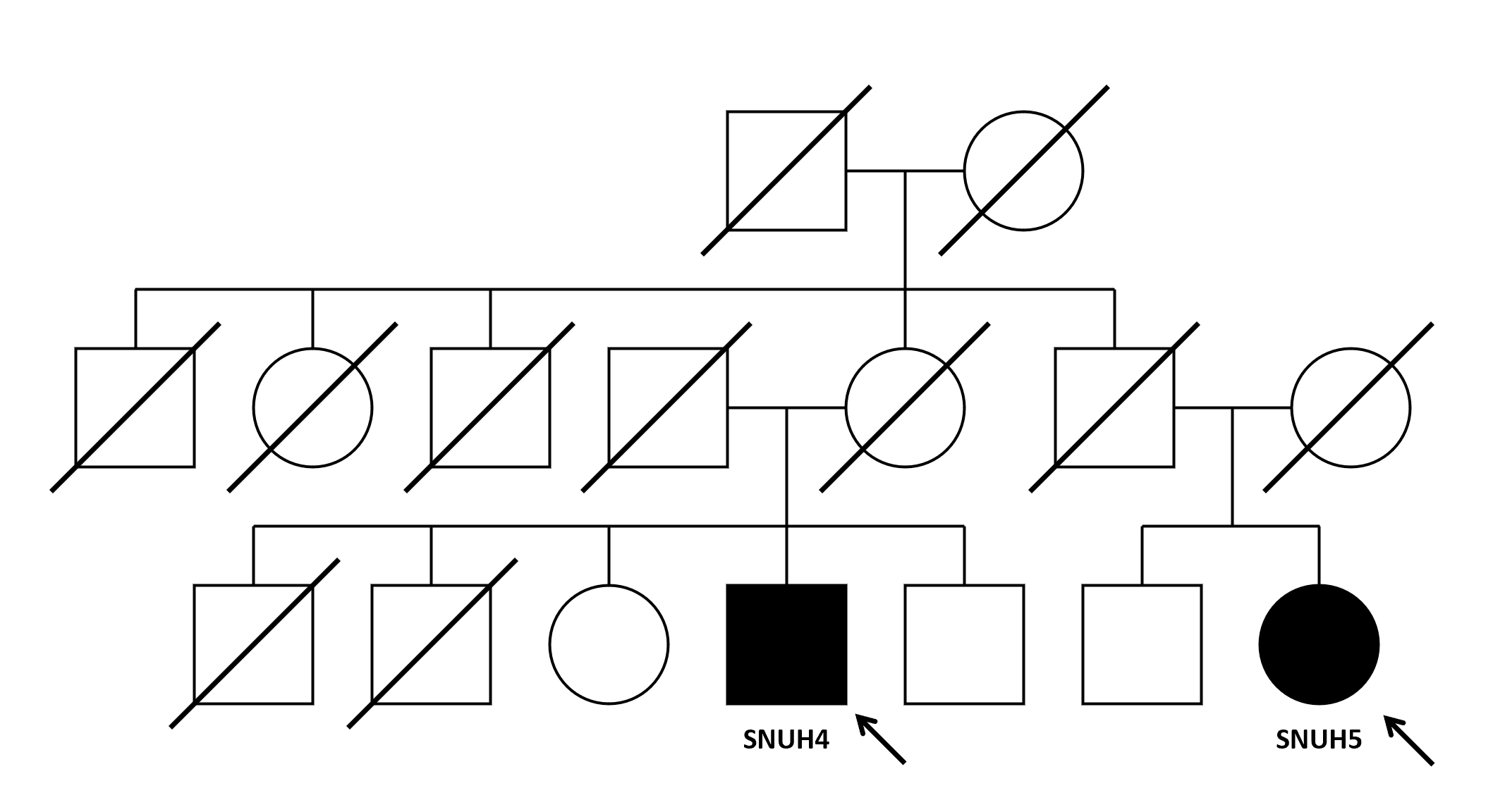
The two patients were first cousins [figure2, patient SNUH4’s mother and patient SNUH5’s father were siblings], and whole-genome sequencing (WGS) was conducted in search for related genes. Unexpectedly, biallelic mutations in ARSA (c.1107+1G>C (splicing) and c1114C>T (p.Arg372Trp)) were found in patient SNUH4 which was sufficient for diagnosis of metachromatic leukodystrophy (MLD). Patient SNUH4 was not MSA by the diagnosis criteria and the two patients were not familial MSA.
Conclusion: Diagnosis of MSA is made based on clinical features with other MSA mimicking diseases being excluded. As there is no definitive diagnostic marker and there are many look-alikes, [5] diagnosis of MSA is challenging. If not for whole-genome sequencing, out patients would have been diagnosed as familial MSA. Therefore, when familial MSA is suspected, extensive work-ups, including comprehensive genetic work-up, should be performed to exclude alternative conditions.
Brain MRI findings
Family tree
References: 1. Wenning GK, Stankovic I, Vignatelli L, et al. The Movement Disorder Society Criteria for the Diagnosis of Multiple System Atrophy. Mov Disord. 2022 Jun; 37(6): 1131-1148.
2. Watanabe H, Shima S, Mizutani Y, et al. Multiple System Atrophy: Advances in Diagnosis and Therapy. J Mov Disord. 2023 Jan; 16(1): 13-21.
3. Itoh K, Kasai T, Tsuji Y, et al. Definite familial multiple system atrophy with unknown genetics. Neuropathology. 2014 Jun; 34(3): 309-13.
4. Soma H, Yabe I, Takei A, et al. Heredity in multiple system atrophy. J Neurol Sci. 2006; 240: 107–110.
5. Kim HJ, Stamelou M, Jeon B. Multiple system atrophy-mimicking conditions: Diagnostic challenges. Parkinsonism Relat Disord. 2016 Jan; 22 Suppl 1: S12-5
To cite this abstract in AMA style:
CH. Jeong, HJ. Kim. Familial Multiple System Atrophy-Mimics: Importance of Comprehensive Evaluation [abstract]. Mov Disord. 2024; 39 (suppl 1). https://www.mdsabstracts.org/abstract/familial-multiple-system-atrophy-mimics-importance-of-comprehensive-evaluation/. Accessed July 3, 2025.« Back to 2024 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/familial-multiple-system-atrophy-mimics-importance-of-comprehensive-evaluation/