Objective: To investigate brainstem structural connectivity changes in REM sleep behavior disorder (RBD) patients using an in-vivo probabilistic brainstem nuclei atlas and 7 Tesla high angular resolution diffusion MR imaging.
Background: RBD is characterized by the absence of REM-sleep muscle atonia. RBD patients have up to 73.5% risk of developing a neurodegenerative synucleinopathy after 12 years from the RBD-diagnosis[1]. Brainstem pathophysiology underlying RBD has been described in animal models, yet it is understudied in living humans due to the lack of an in-vivo brainstem nuclei atlas and to the limited sensitivity of conventional MRI.
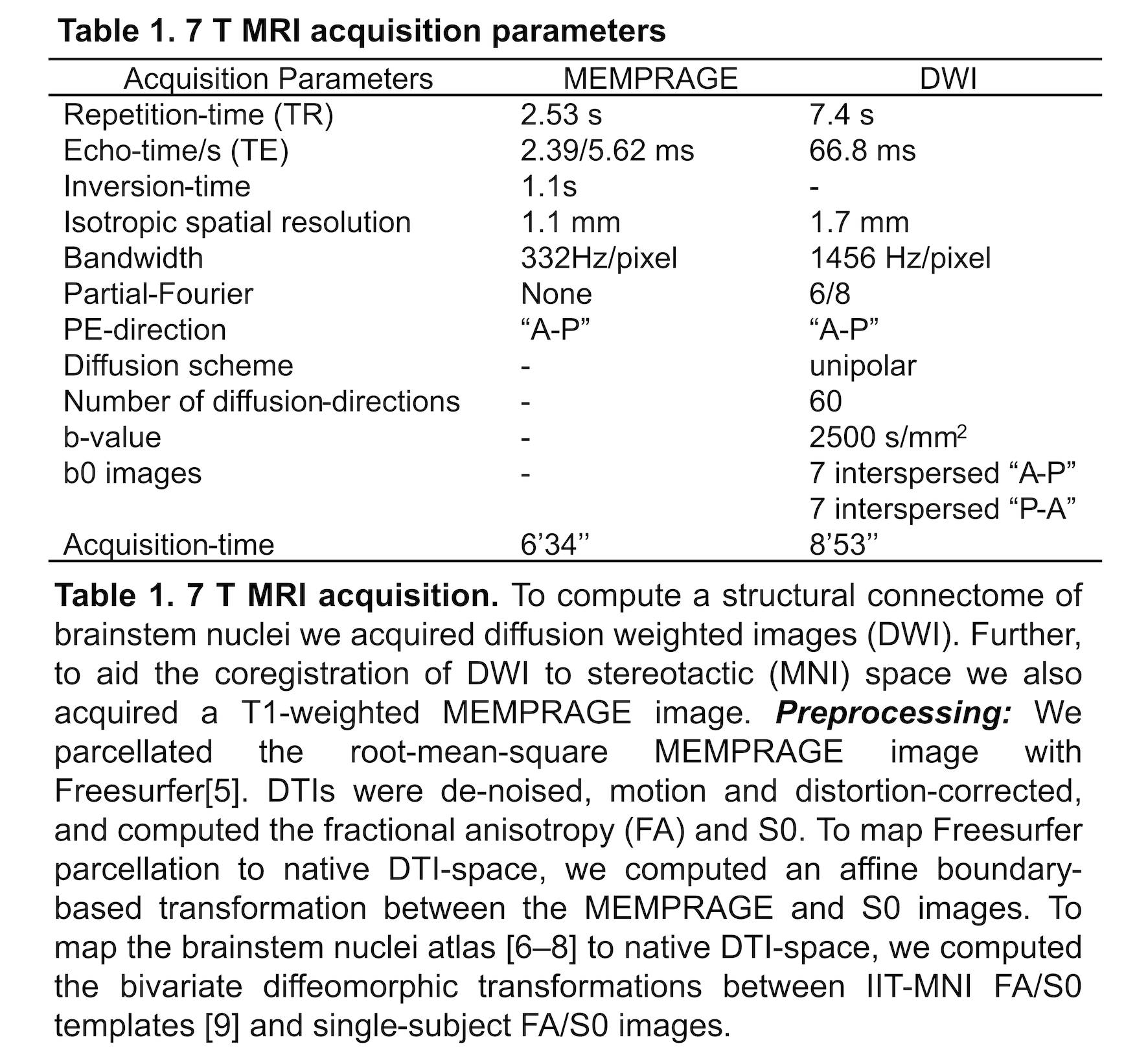
Method: Data acquisition: We performed 7 Tesla MRI [table1] in 12 RBD patients (age: 67.9±1.7 yrs) and 12 controls (age: 66.3±1.6 yrs), under IRB-approval.
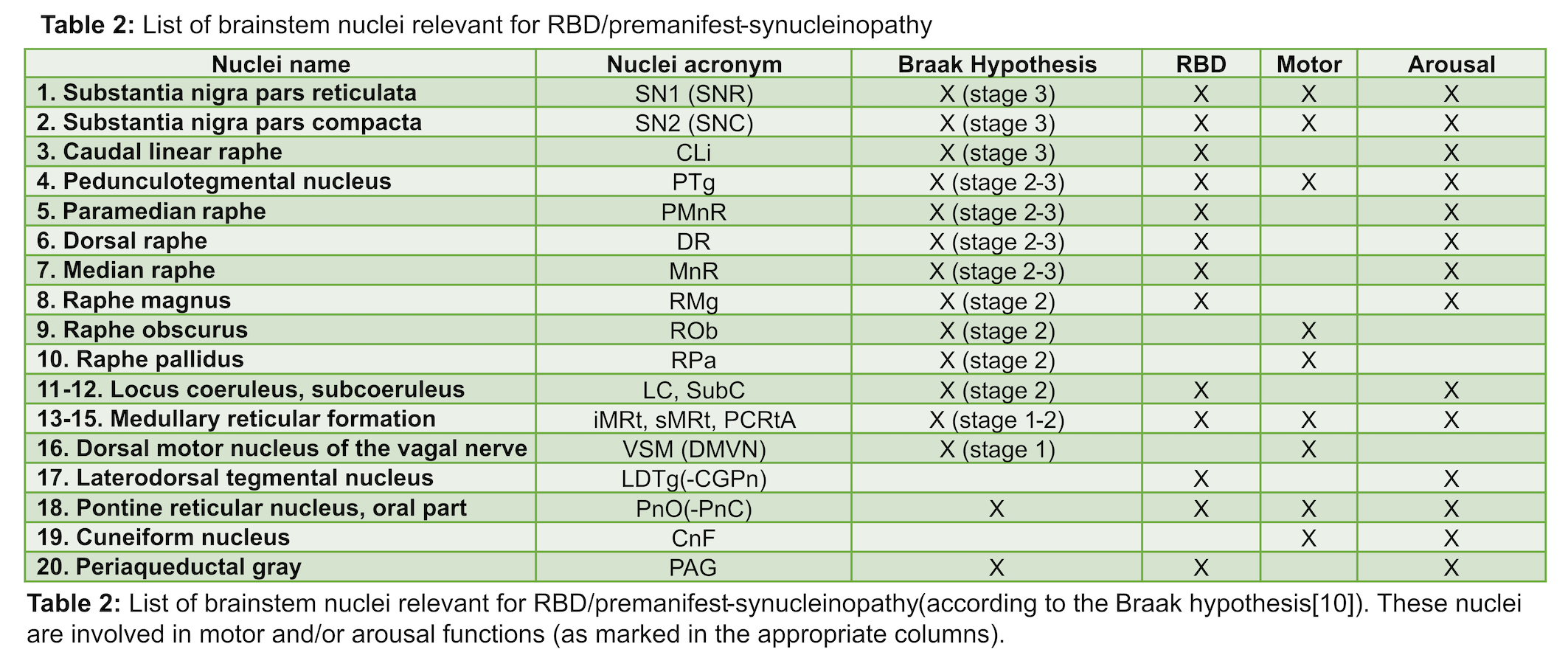
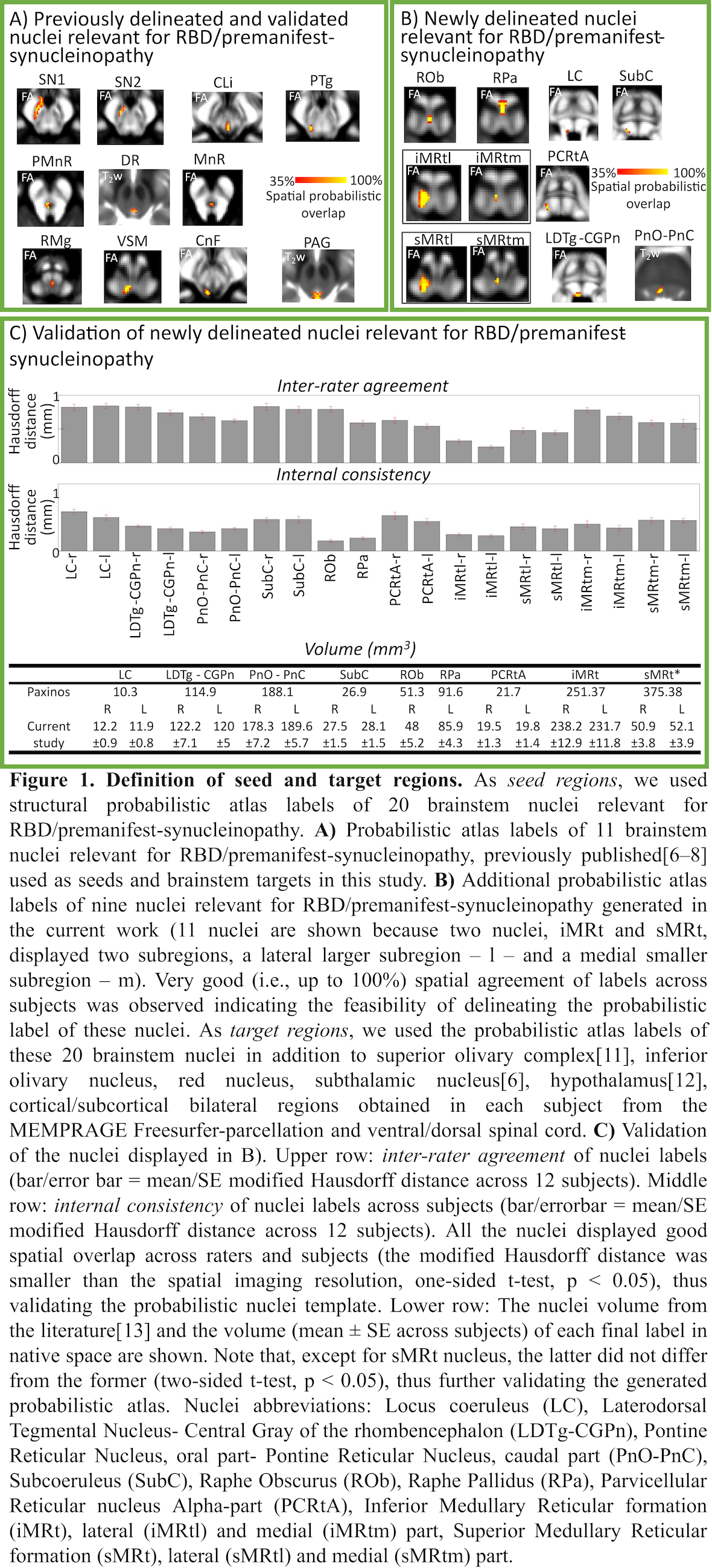
Analysis: a) Preprocessing: see Table 1. b) Definition of seed and target regions for DTI-based connectivity analysis: see Table 2 and Figure 1[table2][figure1]. c) Single-subject and group DTI-based connectivity analysis: We run probabilistic tractography[2]and computed a “structural-connectivity-index” for each pair of seed-target masks (= fraction of streamlines propagated from seed reaching target). d) Statistical analysis: Wilcoxon test was used to compare the differences between groups.
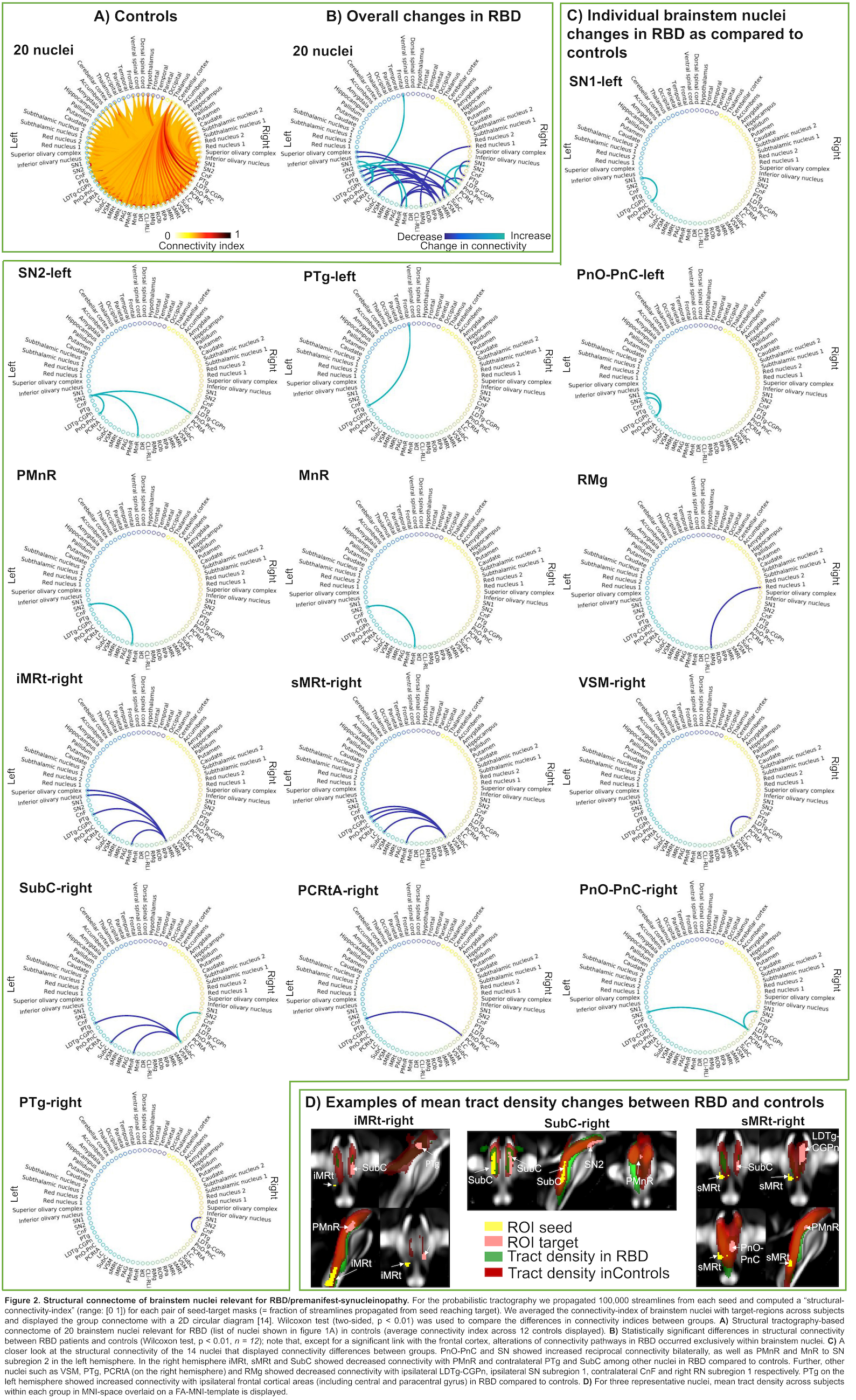
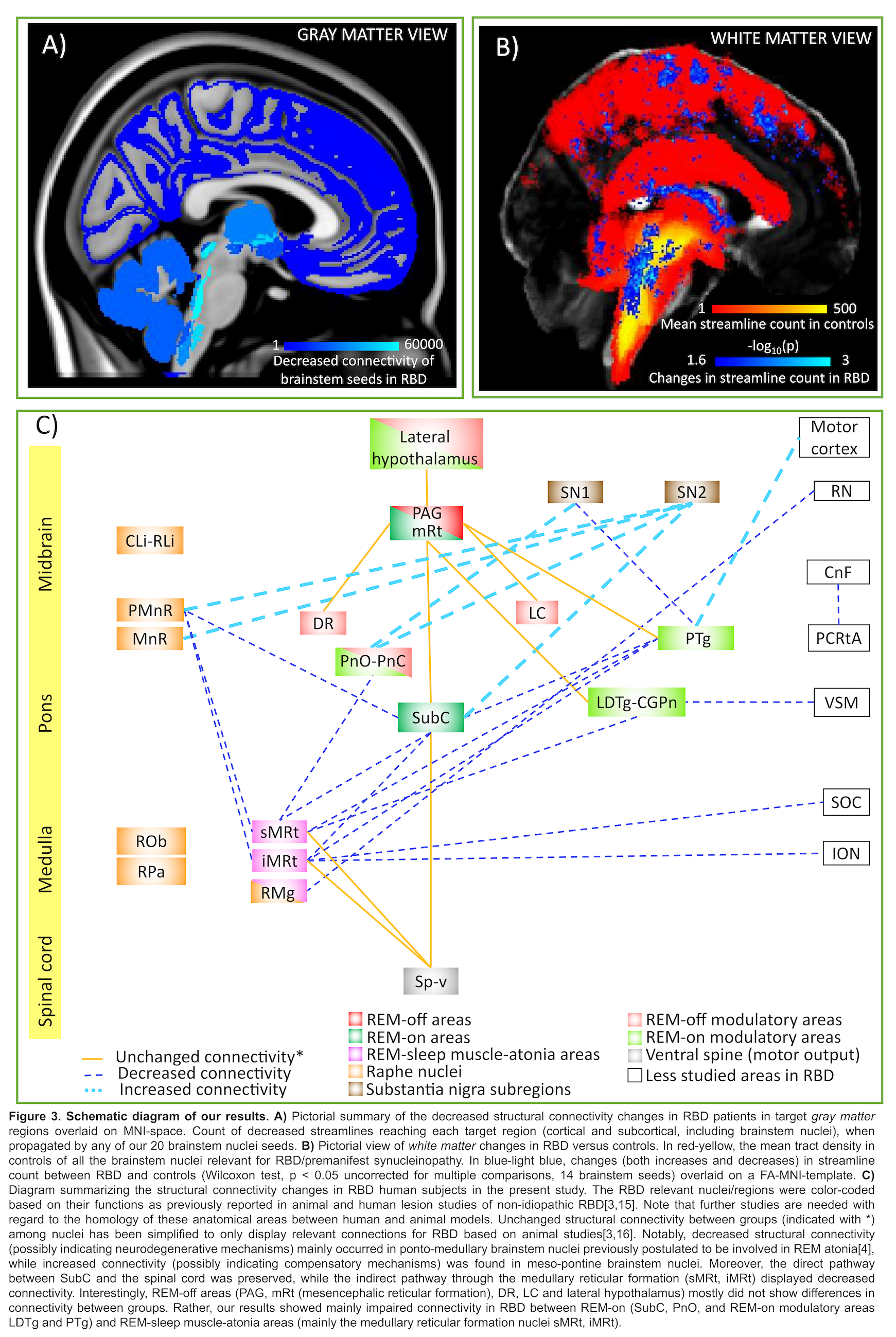
Results: The structural connectome of brainstem nuclei relevant for RBD/premanifest-synucleinopathy showed connectivity changes (specifically in 14 out of 32 brainstem seeds) across groups (Z = 2.6, p < 0.01) mainly within brainstem nuclei[figure2]. Specifically, we found impaired connectivity in RBD between REM-on and REM-sleep muscle-atonia medullary areas. This is in agreement with animal studies showing decreased excitatory connectivity influences between REM-on regions and ventro-medullary nuclei, the latter projecting to spinal motoneurons critical for generating muscle atonia during REM-sleep[3,4]. Most of REM-off areas did not show differences in connectivity between groups. Interestingly, ponto-medullary brainstem nuclei, known to be involved in REM atonia, showed decreased structural inter-connectivity, possibly related to an underlying neurodegeneration process[3]. In contrast, meso-pontine regions showed overall increased inter-connectivity[figure3].
Conclusion: Decreased structural connectivity between REM-on and medullary brainstem nuclei underlies REM-sleep muscle atonia in RBD patients.
References: 1. Postuma, R. B. et al. Risk and predictors of dementia and parkinsonism in idiopathic REM sleep behaviour disorder: a multicentre study. Brain 142, 744–759 (2019). 2. Tournier, J.-D., Calamante, F. & Connelly, A. Robust determination of the fibre orientation distribution in diffusion MRI: non-negativity constrained super-resolved spherical deconvolution. Neuroimage 35, 1459–1472 (2007). 3. Boeve, B. F. et al. Pathophysiology of REM sleep behaviour disorder and relevance to neurodegenerative disease. Brain 130, 2770–2788 (2007). 4. Valencia Garcia, S. et al. Ventromedial medulla inhibitory neuron inactivation induces REM sleep without atonia and REM sleep behavior disorder. Nat Commun 9, 504 (2018). 5. Desikan, R. S. et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31, 968–980 (2006). 6. Bianciardi, M. et al. Toward an In Vivo Neuroimaging Template of Human Brainstem Nuclei of the Ascending Arousal, Autonomic, and Motor Systems. Brain Connectivity 5, 597–607 (2015). 7. Bianciardi, M. et al. A probabilistic template of human mesopontine tegmental nuclei from in vivo 7T MRI. Neuroimage 170, 222–230 (2018). 8. Singh, K. et al. Probabilistic Template of the Lateral Parabrachial Nucleus, Medial Parabrachial Nucleus, Vestibular Nuclei Complex, and Medullary Viscero-Sensory-Motor Nuclei Complex in Living Humans From 7 Tesla MRI. Front. Neurosci. 13, 1425 (2020). 9. Varentsova, A., Zhang, S. & Arfanakis, K. Development of a high angular resolution diffusion imaging human brain template. Neuroimage 91, 177–186 (2014). 10. Braak, H., Ghebremedhin, E., Rüb, U., Bratzke, H. & Del Tredici, K. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 318, 121–134 (2004). 11. García-Gomar, M. G. et al. In vivo Probabilistic Structural Atlas of the Inferior and Superior Colliculi, Medial and Lateral Geniculate Nuclei and Superior Olivary Complex in Humans Based on 7 Tesla MRI. Front. Neurosci. 13, 764 (2019). 12. Pauli, W. M., Nili, A. N. & Tyszka, J. M. A high-resolution probabilistic in vivo atlas of human subcortical brain nuclei. Sci Data 5, 180063 (2018). 13. Paxinos, G., Xu-Feng, H., Sengul, G., & Watson, C. Organization of brainstem nuclei. In: The Human Nervous System. (Elsevier, 2012). 14. Irimia, A., Chambers, M. C., Torgerson, C. M. & Van Horn, J. D. Circular representation of human cortical networks for subject and population-level connectomic visualization. Neuroimage 60, 1340–1351 (2012). 15. Arrigoni, E., Chen, M. C. & Fuller, P. M. The anatomical, cellular and synaptic basis of motor atonia during rapid eye movement sleep: Neural circuitry regulating REM atonia. J Physiol 594, 5391–5414 (2016). 16. Lu, J., Sherman, D., Devor, M. & Saper, C. B. A putative flip–flop switch for control of REM sleep. Nature 441, 589–594 (2006).
To cite this abstract in AMA style:
M. Garcia Gomar, A. Videnovic, K. Singh, M. Stauder, L. Lewis, L. Wald, B. Rossen, M. Bianciardi. New insight into REM-sleep atonia circuits underlying REM sleep behavior disorder in living humans [abstract]. Mov Disord. 2021; 36 (suppl 1). https://www.mdsabstracts.org/abstract/new-insight-into-rem-sleep-atonia-circuits-underlying-rem-sleep-behavior-disorder-in-living-humans/. Accessed February 2, 2026.« Back to MDS Virtual Congress 2021
MDS Abstracts - https://www.mdsabstracts.org/abstract/new-insight-into-rem-sleep-atonia-circuits-underlying-rem-sleep-behavior-disorder-in-living-humans/