Category: Surgical Therapy: Parkinson's Disease
Objective: To describe the protocol of a study evaluating the safety and effectiveness of magnetic resonance-guided focused ultrasound (MRgFUS) pallidothalamic tractotomy (PTT) as a staged bilateral procedure to treat motor complications in patients with idiopathic Parkinson’s disease (PD).
Background: MRgFUS combines a multiple-channel phased-array transducer and MR imaging in a closed-loop procedure for thermal treatment of brain tissue. It utilizes real time thermal feedback and stepwise energy titration, allowing for monitoring of reversible clinical effect and adjustment before lesioning. MRgFUS offers a precise, incisionless method of targeting the pallidothalamic tract (i.e., ansa lenticularis and lenticular fascicles merging at the field of Forel into the thalamic fasciculus) [1]. PTT was introduced with the advantage of creating more consistent and controlled lesions as compared to pallidotomy to maximize the procedure risk/benefit profile [2,3]. MRgFUS PTT yielded high global symptom relief among patients with therapy-resistant PD in two studies from a single centre, 10 of whom were followed for 1 year [4,5].
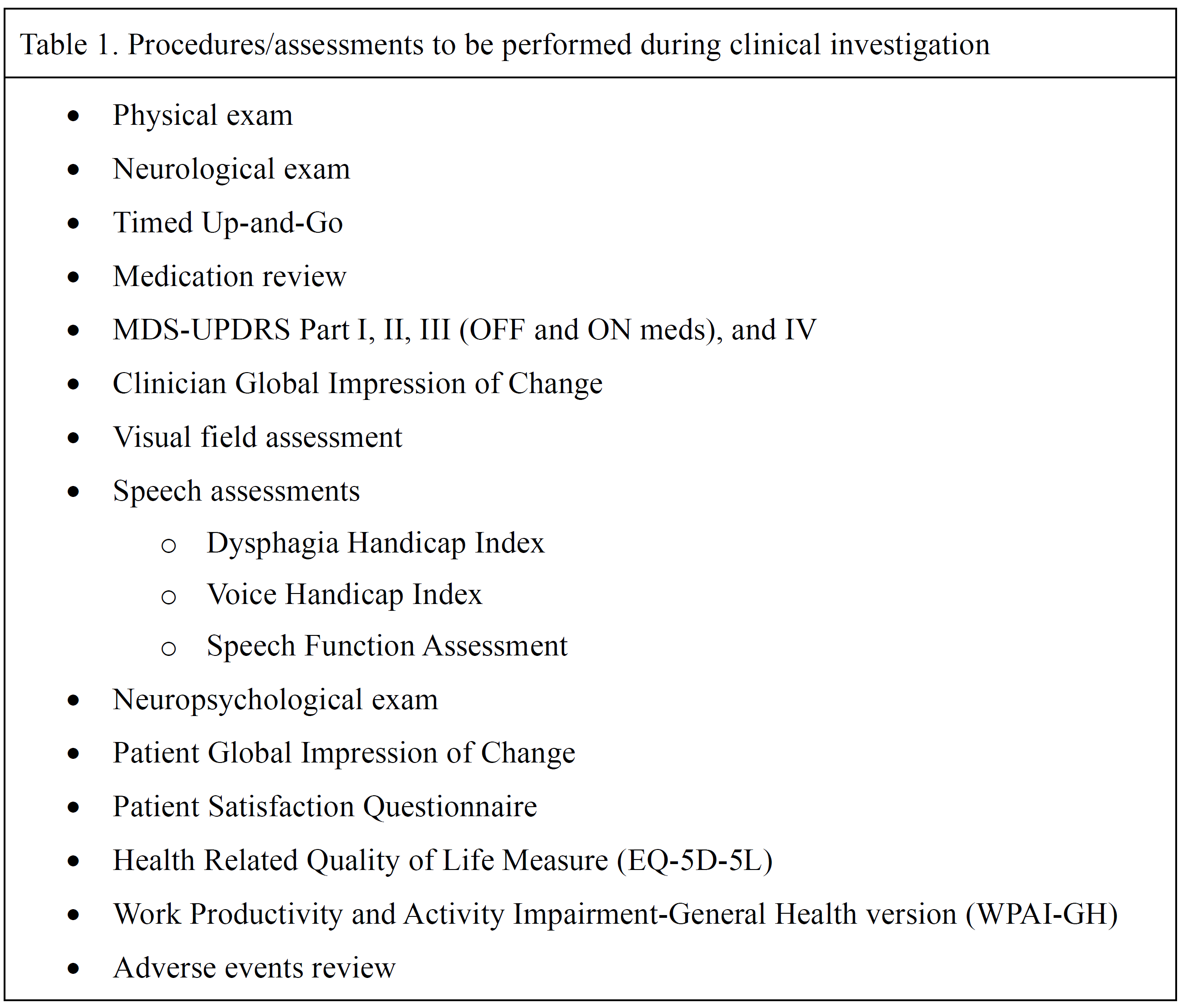
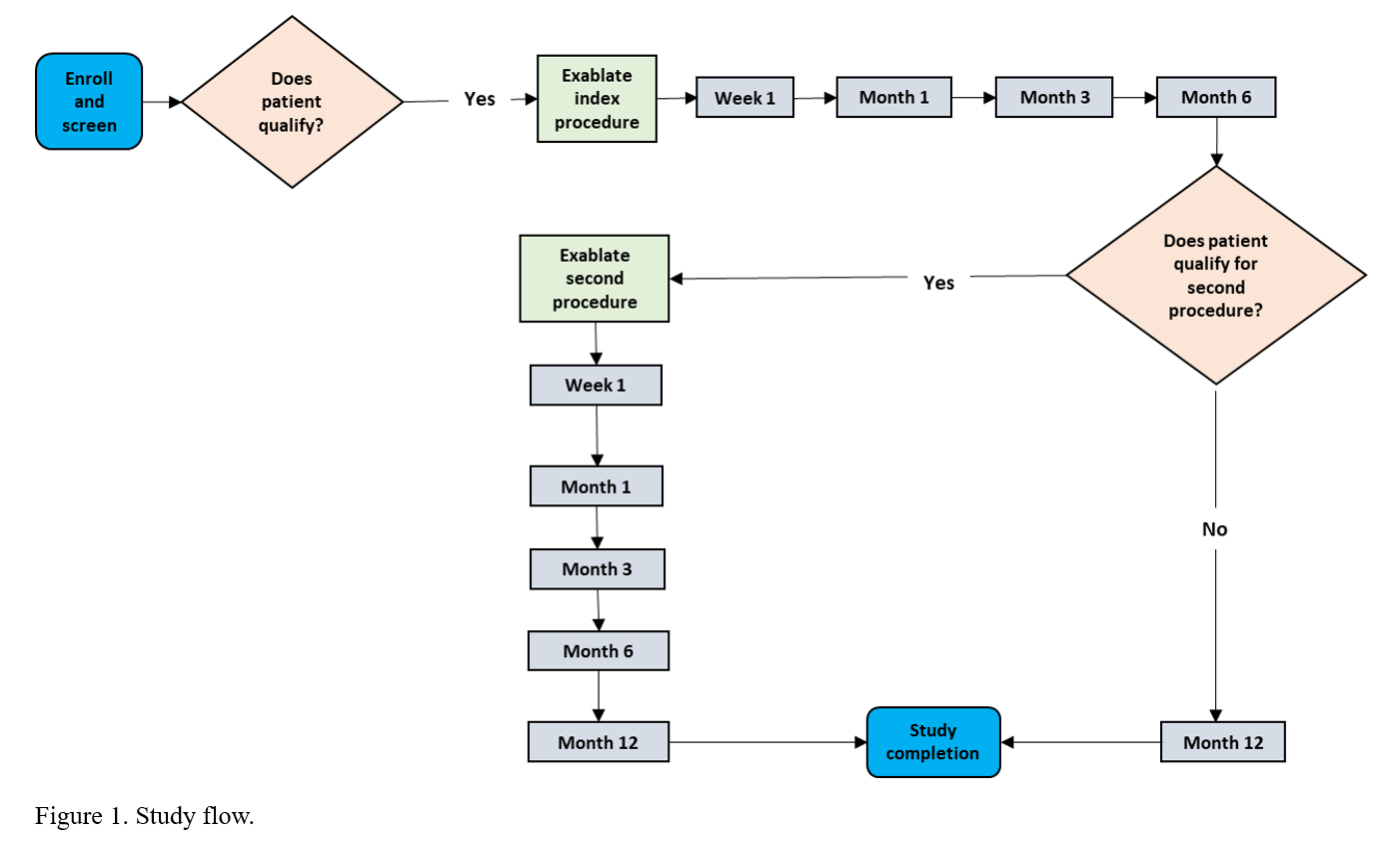
Method: This prospective, open label, single-arm, multicenter clinical trial is enrolling 50-60 patients at up to 10 sites. A minimum of 40 patients are to be treated bilaterally. A unilateral procedure is to be followed by a second procedure, at least 6 months later, in qualifying patients. Follow-up is for 12 months [figure1]. Patients will be enrolled in a separate long-term follow-up protocol through 5 years. Included are levodopa-responsive patients with troubling bilateral motor symptoms. Safety is assessed by the incidence and severity of device- and procedure-related adverse events. The primary efficacy endpoint is the OFF-medication, upper + lower extremity motor score from the Movement Disorder Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS Part III), comparing Month 3 post bilateral treatment to baseline. Multiple procedures/assessments [table1] are to be performed at scheduled clinical visits throughout the study.
Results: The study is registered at NCT04728295. Enrolment is complete and follow-up is in progress.
Conclusion: If successful, the current indication of MRgFUS may expand to include staged bilateral PTT treatment of some patients with idiopathic PD and bilateral motor complications.
Table 1
Figure 1
References: 1. Gallay MN et al. Human pallidothalamic and cerebellothalamic tracts: anatomical basis for functional stereotactic neurosurgery. Brain Struct Funct 2008;212(6):443-463.
2. Aufenberg C et al. A revival of Spiegel’s campotomy: long term results of the stereotactic pallidothalamic tractotomy against the parkinsonian thalamocortical dysrhythmia Thalamus Relat Syst 2005;3(2):121-132.
3. Guridi J et al. Revisiting Forel field surgery. World Neurosurg 2021;147:11-22.
4. Gallay MN et al. MRgFUS pallidothalamic tractotomy for chronic therapy-resistant Parkinson’s disease in 51 consecutive patients: single center experience. Front Surg 2020;6:76.
5. Gallay MN et al. Bilateral MR-guided focused ultrasound pallidothalamic tractotomy for Parkinson’s disease with 1-year follow-up. Front Neurol 2021;12:601153.
To cite this abstract in AMA style:
A. Dalvi, L. Zucker, WC. Chang, PH. Wu, M. Kaplitt, H. Sarva, HM. Eisenberg, P. Fishman, V. Buch, M. Matarazzo, M. Del Alamo, S. Sani, M. Pourfar, A. Mogilner. Safety and effectiveness of staged bilateral MR-guided focused ultrasound pallidothalamic tractotomy for motor complications of Parkinson’s disease: study protocol [abstract]. Mov Disord. 2024; 39 (suppl 1). https://www.mdsabstracts.org/abstract/safety-and-effectiveness-of-staged-bilateral-mr-guided-focused-ultrasound-pallidothalamic-tractotomy-for-motor-complications-of-parkinsons-disease-study-protocol/. Accessed June 30, 2025.« Back to 2024 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/safety-and-effectiveness-of-staged-bilateral-mr-guided-focused-ultrasound-pallidothalamic-tractotomy-for-motor-complications-of-parkinsons-disease-study-protocol/