Session Information
Date: Monday, September 23, 2019
Session Title: Clinical Trials, Pharmacology and Treatment
Session Time: 1:45pm-3:15pm
Location: Agora 3 West, Level 3
Objective: Prove safety and tolerability of allogeneic mesenchymal stem cells (MSC) purified from bone marrow derived from a healthy adult and delivered intravenously in escalated doses to patients with idiopathic Parkinson’s disease (PD).
Background: Chronic neuroinflammation plays a critical role in PD degeneration. MSC exert regenerative and immunomodulatory effects through paracrine and exosome action, by releasing neurotrophic factors, chemokines, cytokines and maintaining immune homeostasis within the neuronal-glial microenvironment.
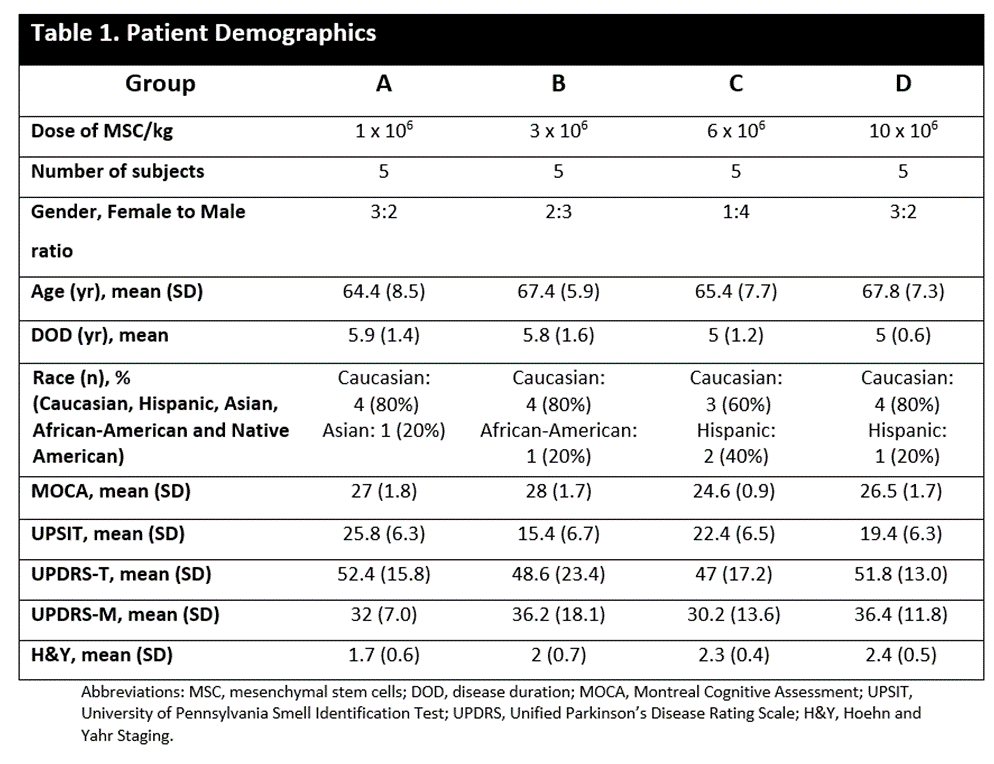
Method: 20 Subjects, 45- 78 years old who met the UK Brain Bank criteria for idiopathic PD (OFF state H&Y of ≤ 3) received one of four IV doses of MSC: 1, 3, 6 or 10 x106 MCS/kg of body weight at CRU, each cohort consists of 5 subjects. The primary outcome of safety is defined as the absence of transfusion reactions, adverse events or organ damage and secondary outcomes by therapy impact on PD progression.
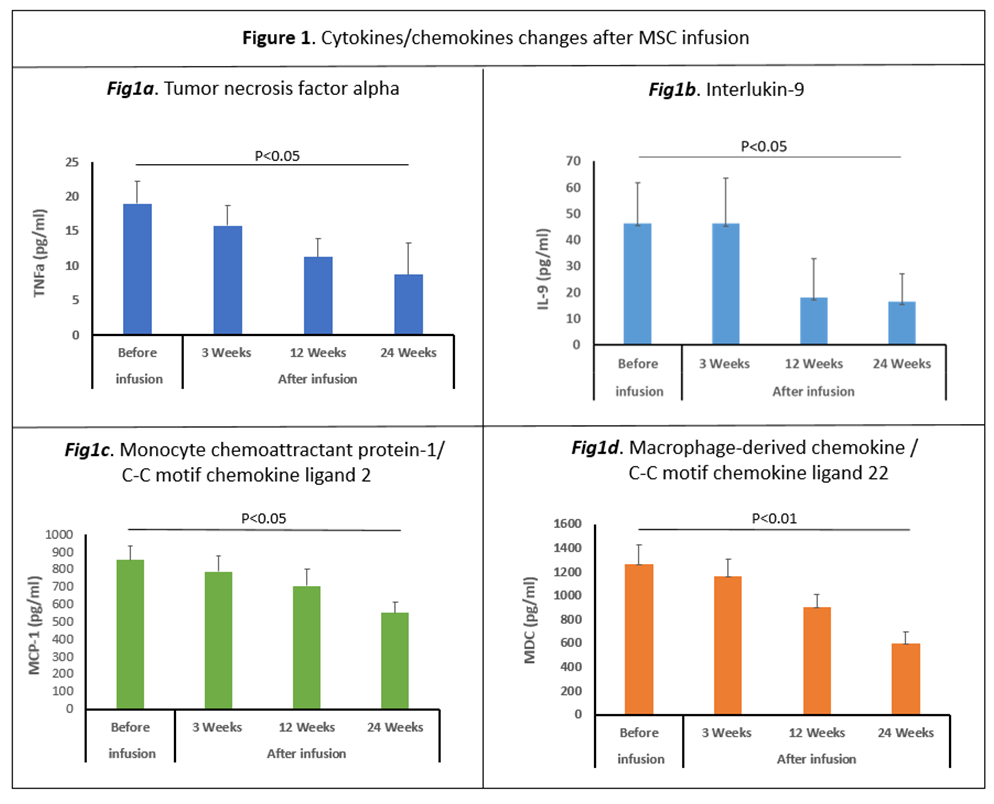
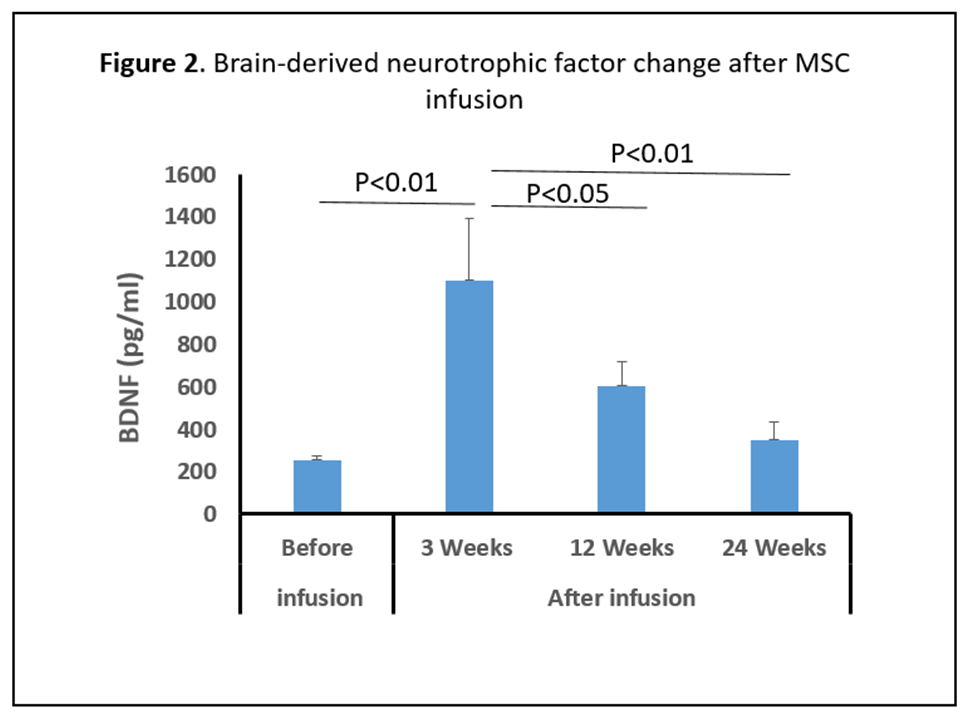
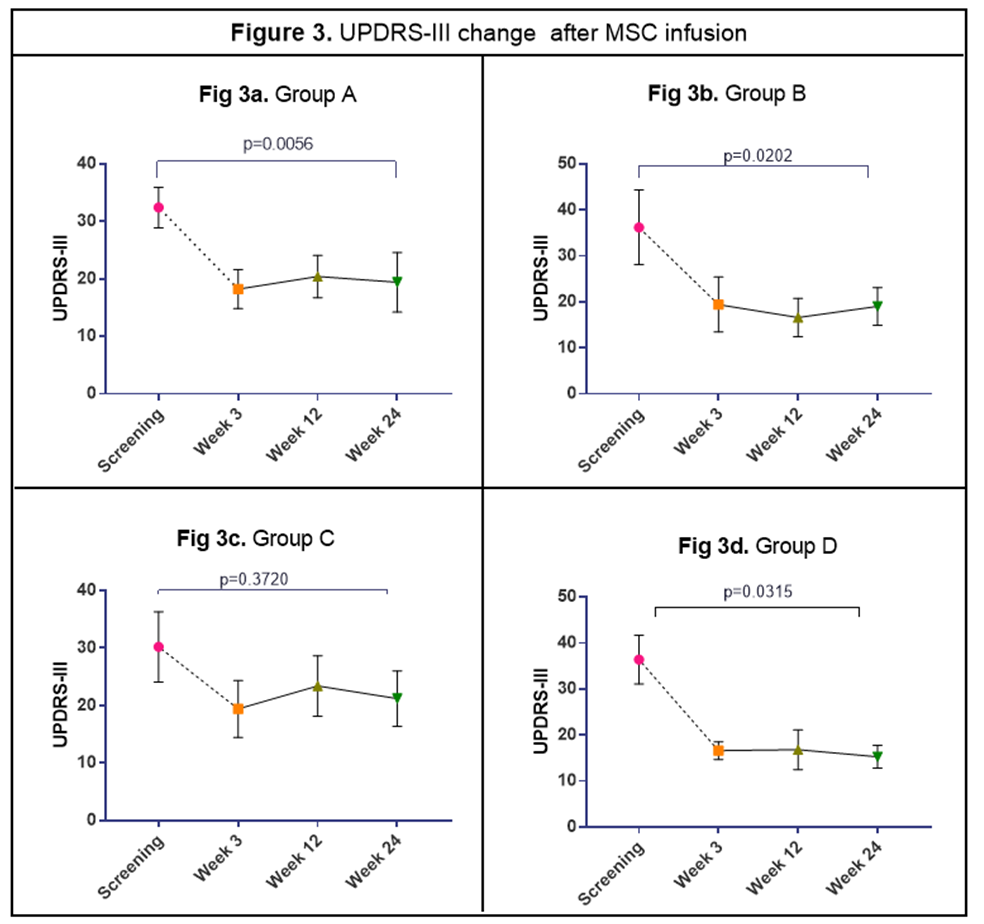
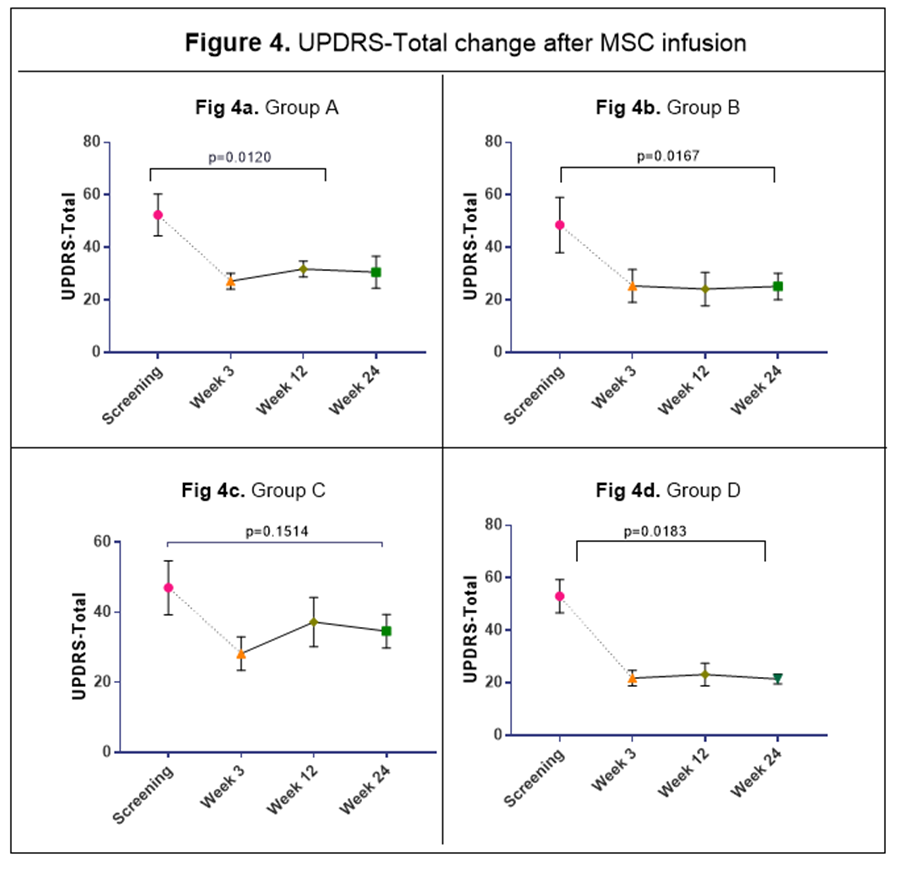
Results: There was no serious adverse reactions in the first 24hrs after a single IV infusion for the 20 subjects; 3 patients had an adverse event (superficial phlebitis, headache, and mild neck pain) during the 24hrs post infusion. At 6-month follow up the most common side effects were hypertension, arthralgia, and nausea (5 patients, 25%). Baseline demographic characteristics in Table 1. Multiple pro-inflammatory molecules significantly decrease over time a) TNF-α levels diminished at 12 wks (33%) and 24 wks (50%); b) MCP-1/CCL-2 and MDC/CCL-22, declined at 12 wks (35%) and 24 wks (48%); and c) IL-9 levels fell at 12 wks (60%) and 24 wks (67%); Figure 1. BDNF increased significantly following MSC infusions at 3wks (340%), 12 wks (140%) and 24 wks (20%), Figure 2. All 20 patients sustained motor improvement (off state) in UPDRS-III, and UPDRS-Total; Figures 3 & 4.
Conclusion: Preliminary findings from this ongoing Phase I study demonstrate that allogeneic MSC infusions appear to be safe and well tolerated at dosages that range from 1 to 10 X 106 MCS/kg in subjects with mild to moderate PD. Our preliminary results warrant the completion of the study and a subsequent phase 2 RCT should determine the efficacy and the right interval/duration of effect of multiple infusions of 10 X 106 MCS/kg. An anti-inflammatory effect with a reduction of chemo-attractive molecules and a significant increase in BDNF levels follows MSC infusions, which may indicate a mechanism of action.
To cite this abstract in AMA style:
M. Schiess, J. Suescun, T. Ellmore, MF. Doursout, E. Furr -Stimming, Z. Mei, A. Gee. Safety of Bone marrow-derived Allogeneic Mesenchymal Stem Cells in Parkinson’s disease Patients [abstract]. Mov Disord. 2019; 34 (suppl 2). https://www.mdsabstracts.org/abstract/safety-of-bone-marrow-derived-allogeneic-mesenchymal-stem-cells-in-parkinsons-disease-patients/. Accessed July 5, 2025.« Back to 2019 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/safety-of-bone-marrow-derived-allogeneic-mesenchymal-stem-cells-in-parkinsons-disease-patients/