Category: Parkinson's Disease: Genetics
Objective: To investigate sleep features among individuals with genetic subgroups of PD, including LRRK2 G2019S-PD, GBA-PD, and iPD (idiopathic PD with no known genetic variant).
Background: PD is a heterogeneous disorder and clinical phenotypes vary in relation to genetic subtype(1,2). Understanding how sleep characteristics differ among two of the most common genetic forms of PD, GBA-PD and LRRK2-PD, and iPD, may inform clinical care.
Method: Actigraphy monitoring(3) evaluated sleep features while awake and asleep for one week. We assessed differences in sleep duration, onset latency, wake after sleep onset, sleep time, sleep efficiency, nap time during the day, sleep start time, and sleep end time in LRRK2 G2019S PD, carriers of the 11 most common GBA variants in the Ashkenazi Jewish population (N370S, L444P, 84GG, IVS2+1, V394L, D409G, A456P, R496H, RecNciI, E326K, T369M), and iPD.
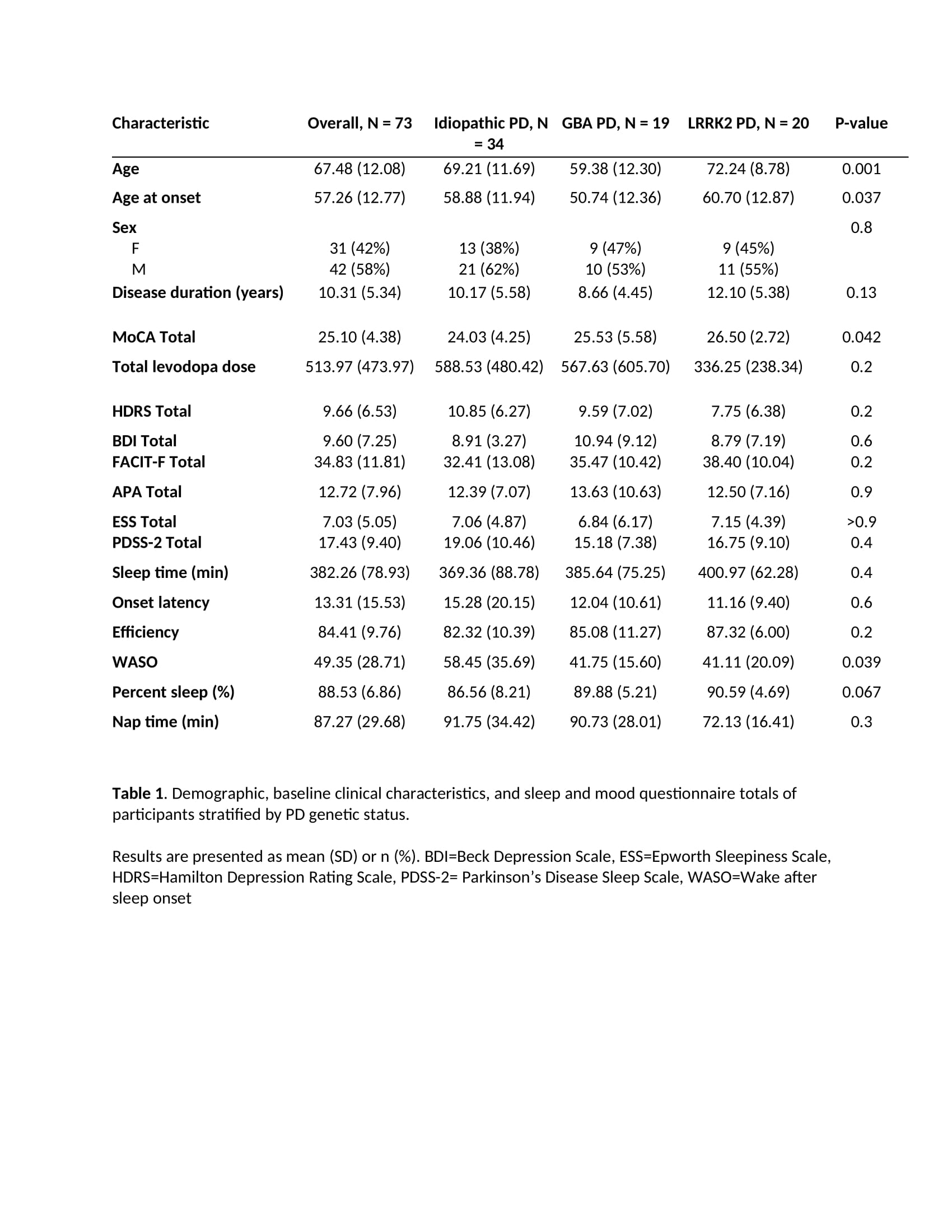
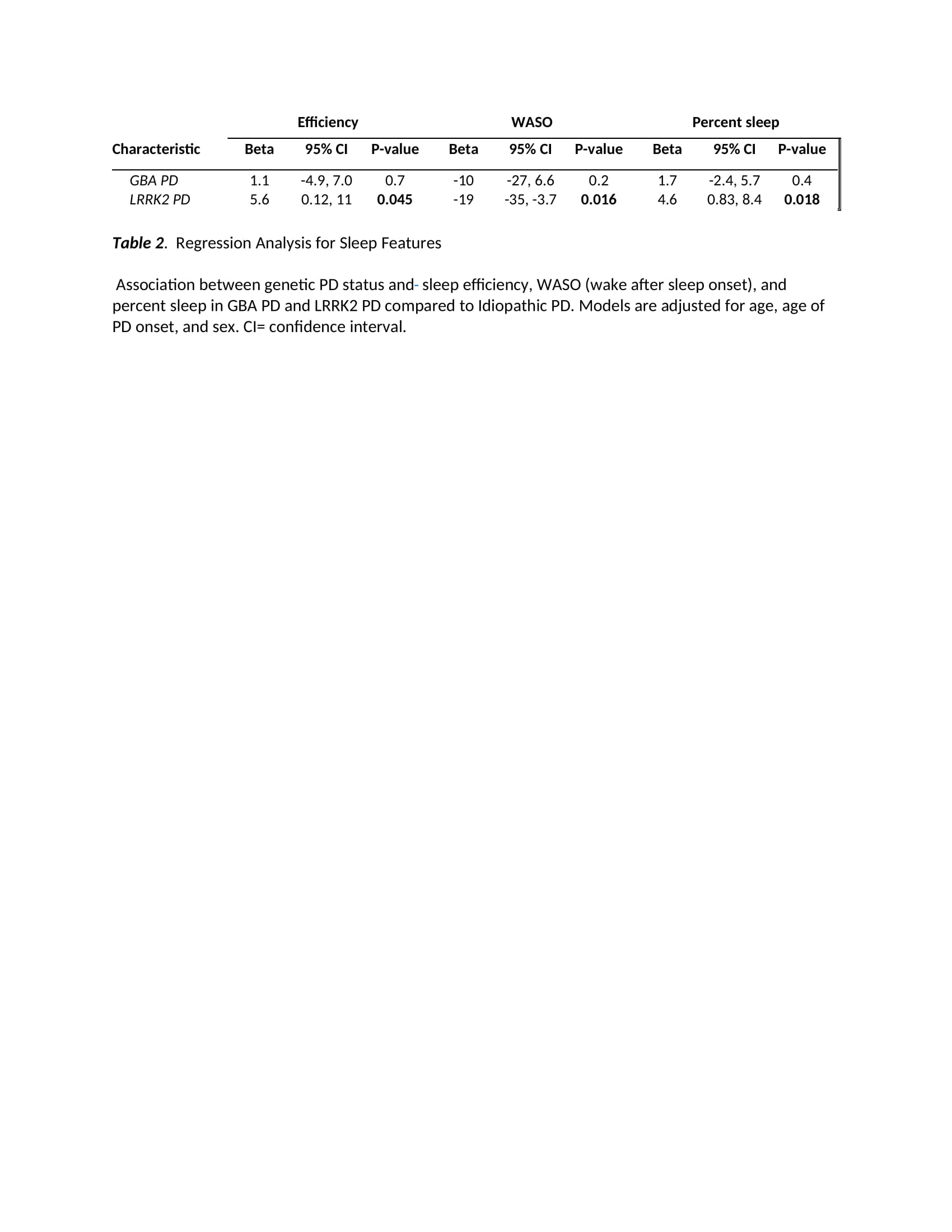
Results: 31 women and 42 men participated: 20 LRRK2-PD and 19 GBA-PD, 34 iPD; baseline disease duration overall, mean 10.3 [SD 5.34] years. LRRK2 were older (72 years [8.8]; GBA-PD: 59[12.3]; iPD: 69[11.7]) with higher age of onset (LRRK2 60.7 [12.9]; GBA 50.7 [12.4]; iPD 59 [11.4]) and MoCA score (LRRK2 26.50 [2.7]; GBA 25.5 [5.6]; iPD 25.1 [4.4], p=.042). In regression analyses adjusting for age, age at onset and sex, LRRK2 status was associated with higher sleep efficiency (5.6%; 95% CI: 0.12, 11, p= 0.046), higher percent sleep (4.6%; 95% CI: 0.83, 8.4, p = 0.018), and lower wake after sleep onset time (-19 minutes; 95% CI: -35, -3.7, p=0.016) relative to iPD. The LRRK2 group also had longer sleep time, shorter sleep latency, less time awake after sleep onset, less nap time and later sleep start time than the iPD and GBA groups; however, these results did not reach statistical significance.
Conclusion: LRRK2-PD participants had more efficient sleep, and higher sleep percent compared to iPD. It is unknown if the sleep differences might be attributable to differences in synuclein burden. Analyses evaluating subjective sleep measures are underway. These findings contribute to our evolving understanding of distinct PD phenotypes.
Table 1
Table 2
References: References:
1. Trinh J, Zeldenrust FMJ, Huang J, Kasten M, Schaake S, Petkovic S, Madoev H, Grünewald A, Almuammar S, König IR, Lill CM, Lohmann K, Klein C, Marras C. Genotype-phenotype relations for the Parkinson’s disease genes SNCA, LRRK2, VPS35: MDSGene systematic review. Mov Disord. 2018 Dec;33(12):1857-1870. doi: 10.1002/mds.27527. Epub 2018 Oct 24. PMID: 30357936.
2.Guadagnolo D., Piane M., Torrisi M. R., Pizzuti A., Petrucci S. (2021). Genotype-phenotype correlations in monogenic Parkinson disease: A review on clinical and molecular findings. Front. Neurol. 12:648588. 10.3389/fneur.2021.648588
3. Natale V, Léger D, artoni M, Bayon V, Erbacci A. The role of actigraphy in the assessment of primary insomnia: a retrospective study. Sleep Med. 2014 Jan;15(1):111-5. doi: 10.1016/j.sleep.2013.08.792. Epub 2013 Nov 15. PMID: 24325809.
To cite this abstract in AMA style:
A. Astefanous, M. Yang, D. Raymond, M. Rawal, J. Liang, A. Cohen, N. Becker, B. Plitnick, A. Yoo, V. Katsnelson, K. Leaver, S. Bressman, M. Figueiro, R. Saunders-Pullman, A. Wise. Sleep Characteristics in Idiopathic and Genetic Parkinson’s Disease [abstract]. Mov Disord. 2024; 39 (suppl 1). https://www.mdsabstracts.org/abstract/sleep-characteristics-in-idiopathic-and-genetic-parkinsons-disease/. Accessed July 5, 2025.« Back to 2024 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/sleep-characteristics-in-idiopathic-and-genetic-parkinsons-disease/