Category: Huntington's Disease
Objective: Review recent literature on time-restricted eating (TRE) in Huntington’s disease (HD) and outline our protocol for an upcoming clinical trial designed to assess the safety, feasibility, and preliminary efficacy of TRE in HD.
Background: HD is a devastating neurodegenerative disorder characterized by motor dysfunction, cognitive impairment, and psychiatric abnormalities that ultimately progress toward death. Effective disease-modifying treatments for HD remain elusive, necessitating exploration of novel therapeutic approaches, including lifestyle modifications that could alter disease progression. Growing evidence suggests that a form of intermittent fasting (IF) known as time-restricted eating (TRE) – defined by consuming all daily caloric intake within a 6-8 hour time window – may slow the progression of neurodegenerative diseases. Although TRE has shown potential in HD animal models and non-HD populations, it has yet to be analyzed for safety, feasibility, and efficacy in persons with HD.
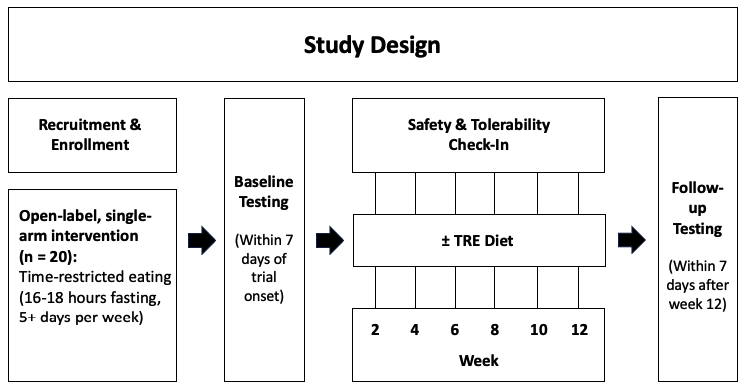
Method: The literature search included articles that examined the effects of any form of dietary fasting protocol in the context of HD (animal or human). Considering the existing evidence, we propose a prospective interventional, open-label, single-arm trial where 25 participants with late prodromal and early manifest HD will be asked to engage in a TRE diet, specifically maintaining a 6-8-hour eating window every day for 12-weeks [figure1]. We will measure body composition, protocol adherence, dietary composition, serum biomarkers, sleep quality, physical activity, mood, and markers of clinical efficacy.
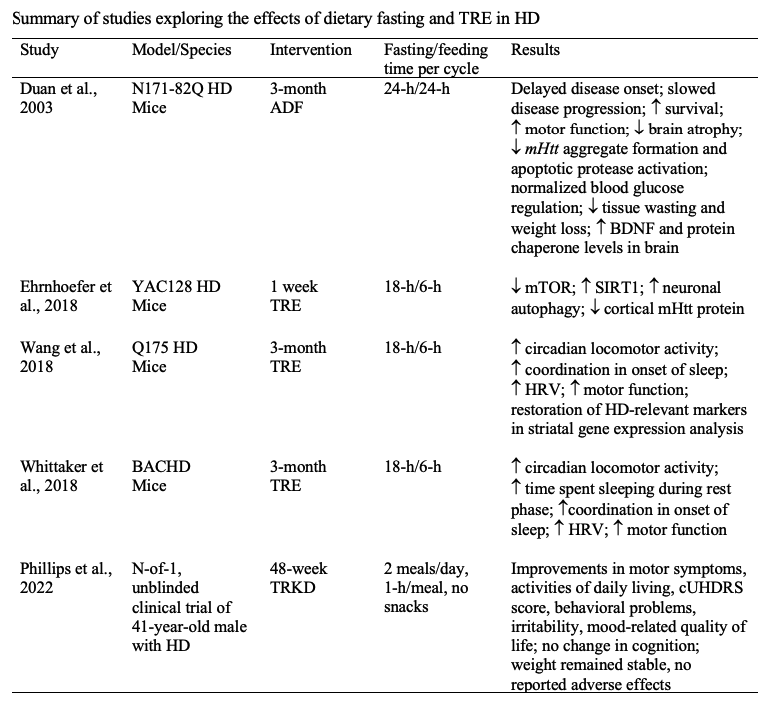
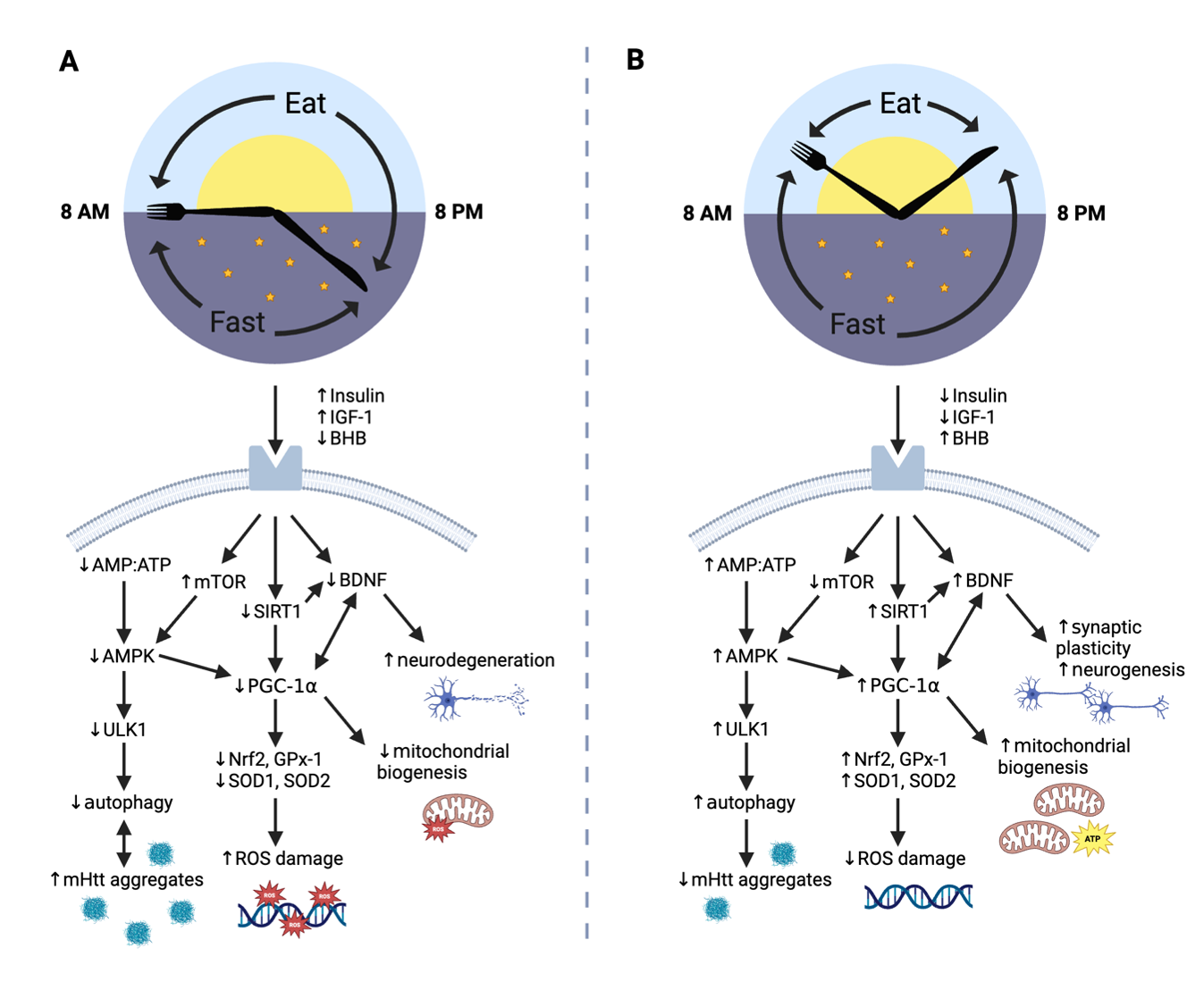
Results: Five studies of TRE in HD were identified in the literature [table1], and the underlying physiologic mechanisms were summarized [figure2]. For our primary endpoints of the proposed trial, we predict at least 20 participants will complete the 12-week study, and we expect to see maintenance of body weight and fat-free mass, adherence to the diet > 80% of the trial days, and an improvement in plasma markers of neurodegeneration from baseline to follow-up.
Conclusion: Using what is known of the TRE mechanism of action and current data, we predict that the diet will be safe, feasible, and may also improve biomarkers of disease progression in persons with HD. We expect this study will lay the foundation for future large-scale clinical trials to evaluate the clinical efficacy of TRE in HD.
Figure 1
Table 1
Figure 2
To cite this abstract in AMA style:
R. Wells, L. Neilson, A. Mchill, A. Hiller. Time-Restricted Eating in Huntington’s Disease: Review of the Literature and a Proposed Clinical Trial [abstract]. Mov Disord. 2024; 39 (suppl 1). https://www.mdsabstracts.org/abstract/time-restricted-eating-in-huntingtons-disease-review-of-the-literature-and-a-proposed-clinical-trial/. Accessed July 5, 2025.« Back to 2024 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/time-restricted-eating-in-huntingtons-disease-review-of-the-literature-and-a-proposed-clinical-trial/