Category: Parkinson's Disease: Genetics
Objective: PLA2G6 is the causative gene for autosomal recessive Dystonia-Parkinsonism (PARK14). This case-control biomarker study aimed to test serum metabolomics of patients diagnosed as PARK14.
Background: Loss of function of PLA2G6 results in dysfunction of organelle like cell membrane, mitochondria and endoplasmic reticulum. In idiopathic Parkinson’s disease (iPD), studies on serum metabolomics have detected that alterations in several metabolic pathways associated with PD disease status and severity of cognitive impairment. Here we assessed the metabolomics profiles in PARK14 patients to unveil biomarkers and related molecular pathways in PLA2G6 mutations.
Method: We enrolled nine PARK14 patients confirmed with homozygous or compound heterozygous PLA2G6 mutations, along with eight healthy one-degree family members of the PARK14 patients (with single heterozygous mutation), and three patients with early-onset Parkinson’s disease. The diagnosis was confirmed according to the diagnostic criteria for Parkinson’s disease (UK PD Society Brain Bank Clinical Diagnostic Criteria or MDS Clinical Diagnostic Criteria for PD) and genetic testing. Eight healthy age and sex-matched subjects were recruited as controls. We analyzed all participants’ serum using well-established metabolomics technologies, UHPLC-QTOF/MS analysis. The pathogenicity of PLA2G6 mutations was evaluated according to the ACMG Guideline and confirmed by the enzyme activity measured in skin fibroblasts.
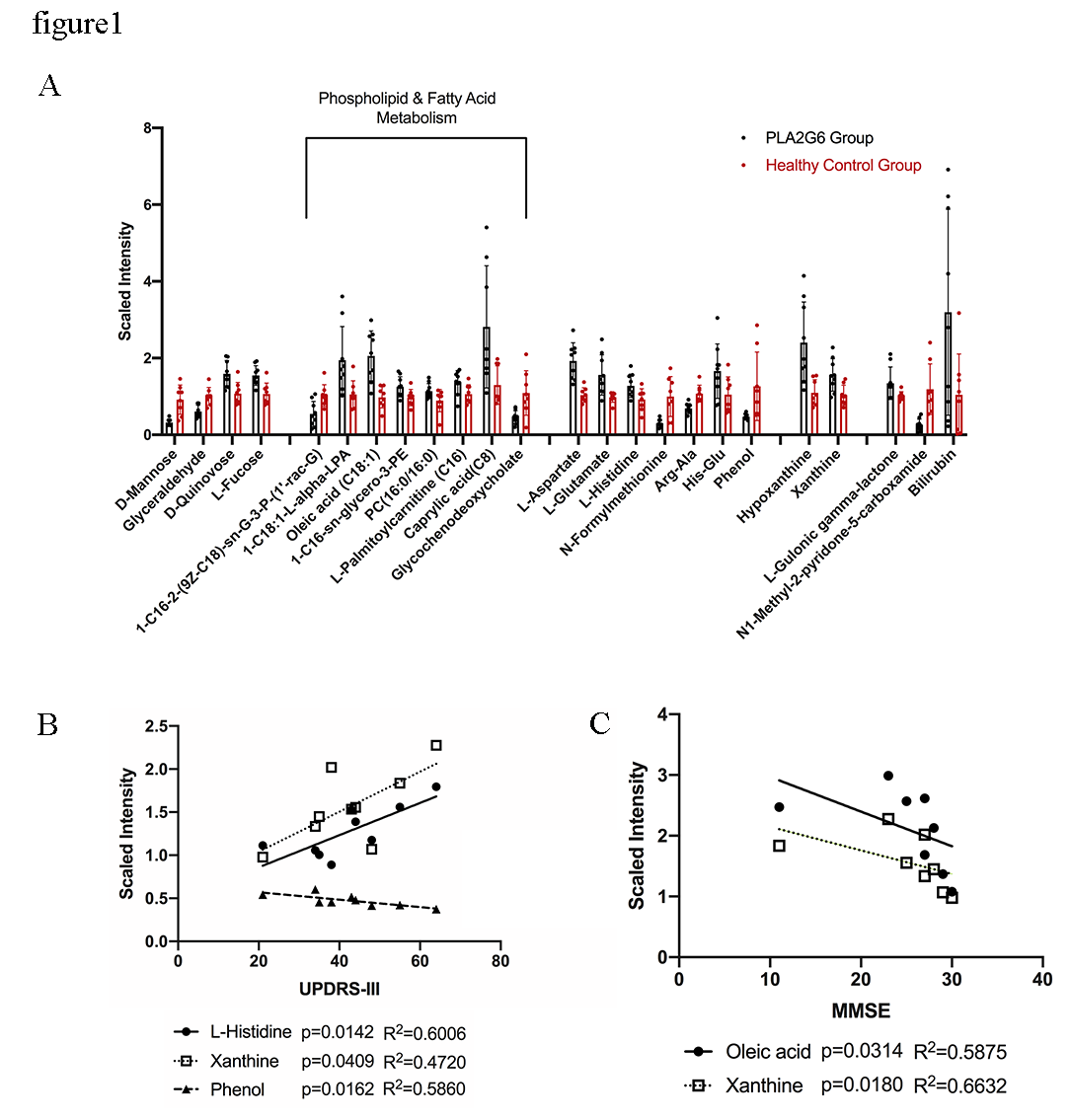
Results: Profiles from PARK14 patients showed significantly higher levels of oleic acid (C18:1), palmitic acid (C16:0) and their derivatives, and a reduced concentration in the rest fatty acid species (figure1A). Products of hyperoxidative stress, energy depletion and cell damage (Xanthine, L-Histidine) enhanced significantly in PARK14 group and were highly associated with UPDRS-III and MMSE scores (figure1B-C). Metabolites alterations in serum had good predictive accuracy for PARK14 diagnose (AUC 0.903) and advanced stage in PARK14 (AUC 0.944).
Conclusion: The significantly altered metabolites can be used to differentiate PLA2G6 pathogenic mutations and predict disease severity. Also, the abnormally increased C18:1 level in PARK14 patients might suggest that PLA2G6 dysfunction had unique pathogenic mechanism in lipid metabolism to iPD.
To cite this abstract in AMA style:
C. Chen, M. Lou, Y. Sun, W. Wang, J. Wang. Serum Metabolomic Characterization of PLA2G6-associated Dystonia-Parkinsonism: a case-control biomarker study [abstract]. Mov Disord. 2022; 37 (suppl 2). https://www.mdsabstracts.org/abstract/serum-metabolomic-characterization-of-pla2g6-associated-dystonia-parkinsonism-a-case-control-biomarker-study/. Accessed December 27, 2025.« Back to 2022 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/serum-metabolomic-characterization-of-pla2g6-associated-dystonia-parkinsonism-a-case-control-biomarker-study/