Category: Parkinson’s Disease: Clinical Trials
Objective: To evaluate recruitment, eligibility and characteristics of clinic referred vs self-referred patients in the ongoing Trial of Parkinson’s and Zoledronate (TOPAZ, NCT03924414)
Background: People with neurodegenerative parkinsonism have a 4-fold increased fracture risk, but few receive preventive treatment. To reduce barriers to treatment, TOPAZ is a completely home-based, double blind, placebo-controlled, randomized clinical trial evaluating the efficacy and safety of the FDA-approved bisphosphonate zoledronate in preventing fractures in older adults with neurodegenerative parkinsonism. Patients are identified from clinics and community sources.
Method: Recruitment sources include movement disorder clinics (“clinic referred”), or self-referral after learning of TOPAZ via community outreach (i.e. support groups, social media, etc.), advocacy group outreach, Parkinson’s disease (PD) registry mailings, webinars, participant peer recruitment or paid online recruiting services (“self-referred”). Interested participants or their legally authorized representatives review study information and give consent online (https://www.topazstudy.org). After initial screening, diagnostic eligibility is determined by movement disorders experts using medical records and/or telemedicine. Research nurses conduct safety assessments, patients are randomized and study drug is administered in the home. Fractures are ascertained q 4 months for up to 5 years by email or telephone and adjudicated by expert review.
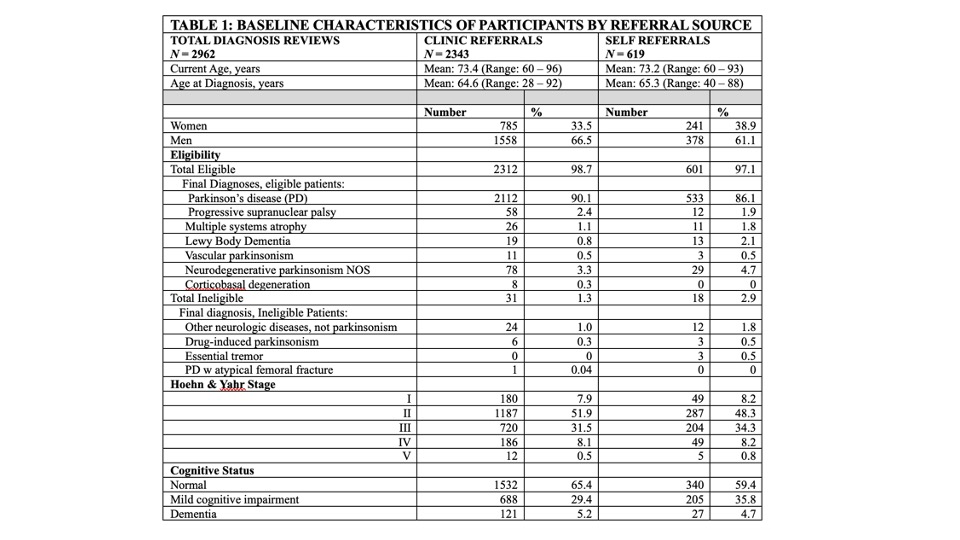
Results: As of 3/11/24, 5,232 persons have registered on the website, 3,833 consented, 2,962 passed initial screening and have had expert diagnostic evaluation. Diagnostic eligibility is high (98.3%). PD is the most common diagnosis (89%), followed by progressive supranuclear palsy (3.4%) [Table 1]. Patients with greater parkinsonism severity and those with dementia enrolled via both clinic and self-referral [Table 1]. Of 1922 randomized, 95% are actively in follow-up (up to 48 months), 4% died, 1% discontinued follow-up.
Conclusion: Remote, online/home-based enrollment, eligibility and safety evaluations, randomization, treatment and follow-up are successful. Diagnostic eligibility is high whether recruitment is through clinic or self-referral via community sources. Patients with greater severity of parkinsonism and those with cognitive impairment are represented.
Table 1
To cite this abstract in AMA style:
C. Tanner, N. Luthra, S. Goldman, R. Zuzuarregui, E. Brown, C. Meng, J. Perkins, T. Hue, C. Schambach, D. Kreisel, D. Young, I. Bledsoe, L. Racelo, P. Nanda Kumar, P. Ranola, M. Schwarzschild, R. Dorsey, A. Espay, S. Krischer, J. Falcon, C. Comella, M. Siddiqui, F. Gao, M. Ledoux, E. Byrd, I. Litvan, N. Mcfarland, K. Mitchell, D. Standaert, J. Beck, K. Williams, M. Drake, D. Bauer, K. Lyles, S. Cummings. Clinic vs Self-Referral for a Home-Based Fracture Prevention Trial of Zoledronate vs Placebo in Patients with Neurodegenerative Parkinsonism [abstract]. Mov Disord. 2024; 39 (suppl 1). https://www.mdsabstracts.org/abstract/clinic-vs-self-referral-for-a-home-based-fracture-prevention-trial-of-zoledronate-vs-placebo-in-patients-with-neurodegenerative-parkinsonism/. Accessed July 12, 2025.« Back to 2024 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/clinic-vs-self-referral-for-a-home-based-fracture-prevention-trial-of-zoledronate-vs-placebo-in-patients-with-neurodegenerative-parkinsonism/