Objective: To assess the precision of Deep Brain Stimulation (DBS) electrode placement within Subthalamic nucleus (STN) using Advanced Imaging and artificial intelligence, considering the challenges posed by brain shift in immediate post-implantation imaging
Background: The efficacy of Deep Brain Stimulation (DBS) depends on precise lead placement within the STN. Brain shifting is often associated with cerebrospinal fluid loss and pneumocephalus. Here, we present two cases where DBS planning software’s AI erroneously indicated electrode misplacement due to brain shifting. However, a repeat MRI imaging after two weeks, the same software indicated an accurate placement.
Method: Case -1:
A 48-year-old male with Parkinson’s disease (PD) underwent STN DBS using Boston Genus leads. Initial postoperative CT fusion scans with STIMVIEW XT suggested DBS lead misplacement beyond STN boundaries. CT brain revealed left frontal pneumocephalus. An MRI brain after 2 weeks showed good positioning within STN and significant motor improvement with no adverse events.
Case – 2:
A 62-year-old lady with PD underwent STN DBS using Boston Genus leads. Initial postoperative CT fusion scans with STIMVIEW XT suggested DBS lead misplacement beyond STN boundaries. CT brain showed pneumocephalus. An MRI brain after 2 weeks showed good positioning within STN and significant motor improvement with no adverse events.
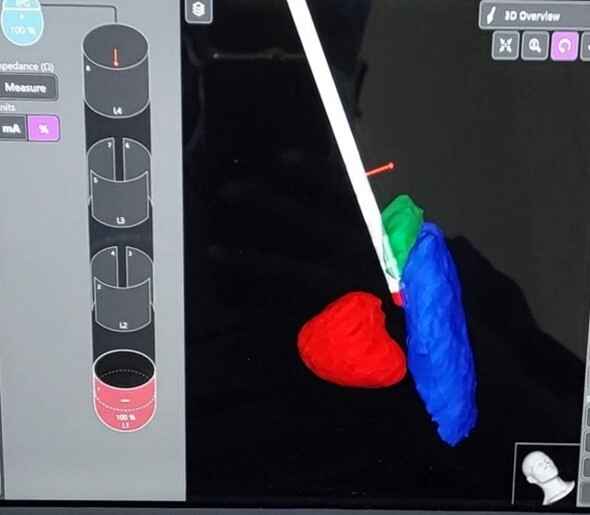
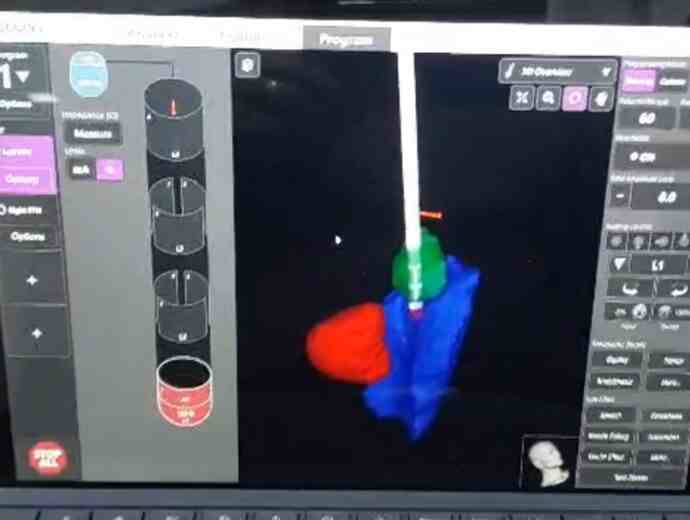
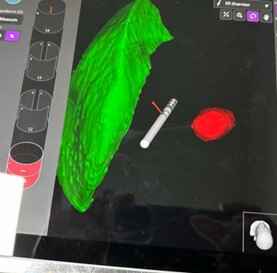
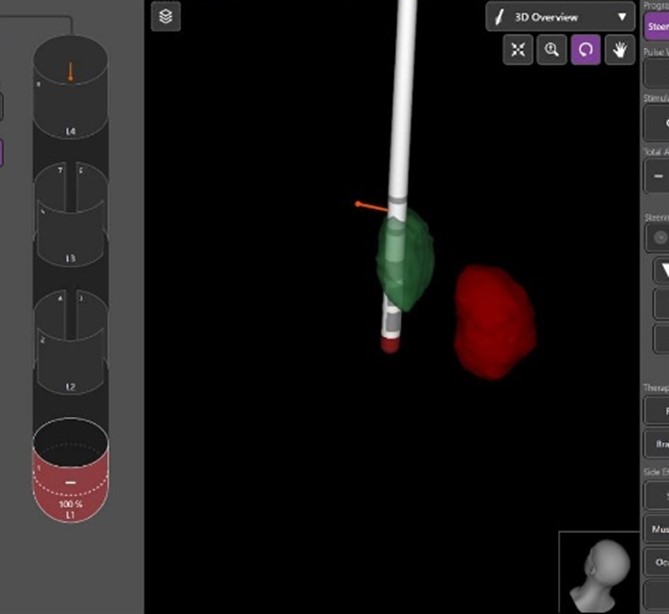
Results: The advancements in imaging techniques and surgical procedures aim to enhance the accuracy and safety of DBS electrode placement. The Boston STIMVIEW XT software automatically identifies electrodes in post-operative CT brain images and allows clinicians to visualize the lead placement and model brain anatomy during the programming process. The software indicated notable radial errors, implying misplacement. Nevertheless, subsequent MRI scans, done two weeks later, after the pneumocephalus resolution, were used for the 3D visual reconstructions to confirm correct electrode placement within the target area. The patient’s clinical improvement, coupled with the absence of adverse effects, further affirmed precise electrode positioning.
Conclusion: Our findings highlight the dynamic nature of postoperative pneumocephalus, which can impact the interpretation of imaging data and computational algorithms. By recognizing and addressing these factors, we can avoid unnecessary concern among the intervention team.
Image -1: misplaced leads in first imaging
Image -2:properly placed leads post op imaging
Image -3: misplaced leads in first imaging
Image -4: properly placed leads post op imaging
References: 1. Okun, M. S. et al. Management of referred deep brain stimulation failures: A retrospective analysis from two movement disorder centers: 818. Neurosurgery 57(2), 401
2. Rolston, J. D., Englot, D. J., Starr, P. A. & Larson, P. S. An unexpectedly high rate of revisions and removals in deep brain stimulation surgery: Analysis of multiple databases. Parkinsonism Relat. Disord. 33, 72–77 (2016).
3. Dembek TA, Hoevels M, Hellerbach A, et al. Directional DBS leads show large deviations from their intended implantation orientation. Parkinsonism Relat Disord. 2019;67:117–1211.
To cite this abstract in AMA style:
V. Paramanandam, R. Alugolu, A. Sukumaran, R. Kandadai, R. Borgohain. Deep brain stimulation Intraoperative imaging: False errors in the era of artificial intelligence [abstract]. Mov Disord. 2024; 39 (suppl 1). https://www.mdsabstracts.org/abstract/deep-brain-stimulation-intraoperative-imaging-false-errors-in-the-era-of-artificial-intelligence/. Accessed July 2, 2025.« Back to 2024 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/deep-brain-stimulation-intraoperative-imaging-false-errors-in-the-era-of-artificial-intelligence/