Category: Parkinson's Disease: Neuroimaging
Objective: To identify unique subtypes of synucleinopathy with distinct spatiotemporal patterns and trajectories of brain atrophy and disease burden.
Background: The synucleinopathies manifest as a spectrum of disorders that vary in features and severity, ranging from idiopathic/isolated REM sleep behavior disorder (iRBD) in the prodromal phase to dementia with Lewy bodies (DLB) and Parkinson’s disease (PD) in the overt phase. Patterns of brain atrophy in iRBD are already reminiscent of what is later seen in overt disease; however, how brain atrophy begins and progresses remains unclear. To better understand the inter-individual variability in iRBD, a systematic investigation of the sequential brain changes leading to overt disease is needed.
Method: A multicentric cohort of 538 subjects with synucleinopathies (n=451 iRBD and n=87 DLB) recruited from 11 international study centers underwent T1-weighted MRI imaging and clinical assessment. Scans underwent vertex-based cortical surface reconstruction and volumetric segmentation to quantify brain atrophy. We next applied the novel unsupervised machine learning algorithm, Subtype and Stage Inference (SuStaIn), to stratify subjects into distinct spatiotemporal patterns of brain atrophy and correlated the distinct subtypes with clinical markers of disease progression.
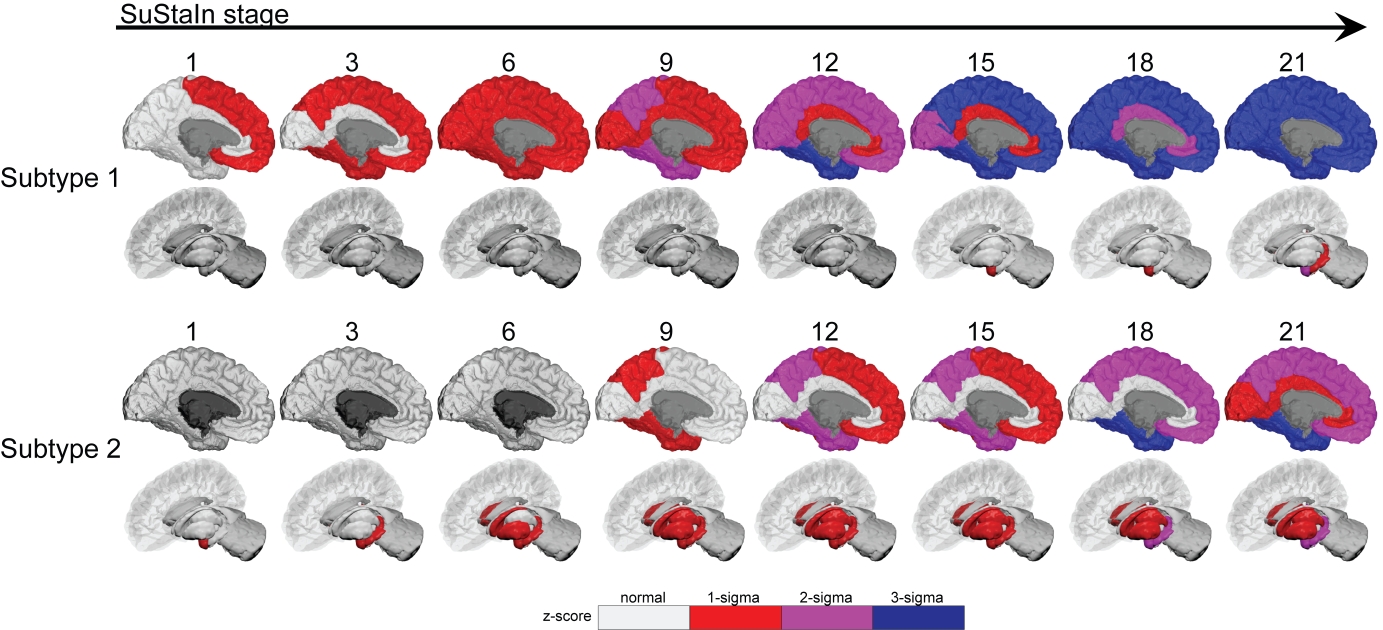
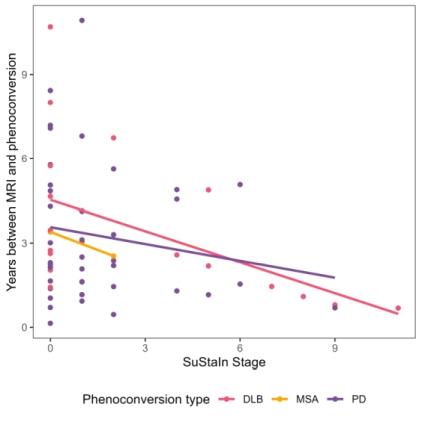
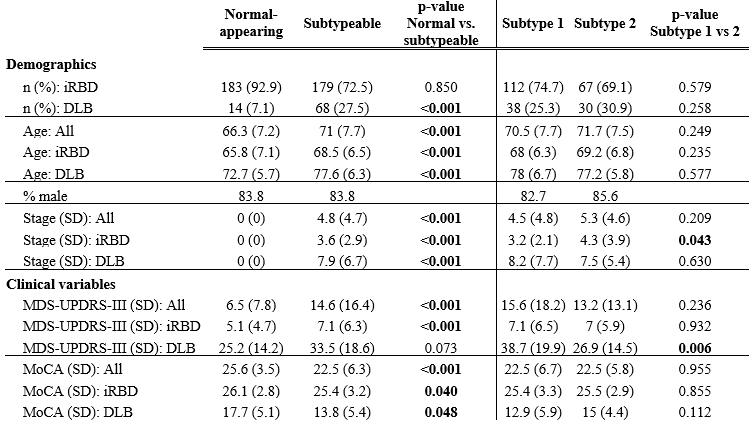
Results: SuStaIn identified two unique subtypes of brain atrophy progression: (i) a “cortical-first” progression subtype characterized by atrophy beginning in the frontal lobes followed by the temporal and parietal areas and remaining cortical areas, with the involvement of subcortical structures at later stages; and (ii) a “subcortical-first” progression, which involved atrophy beginning in the limbic areas, then basal ganglia, and only involving cortical structures at late stages [figure1]. Subjects that were not classifiable (“normal-appearing”) had lower motor and cognitive disease burden compared with those that were subtypeable [table1]. Finally, later disease stages were associated with more imminent phenoconversion of iRBD subjects to overt disease [figure2].
Conclusion: Patients with synucleinopathy can be classified into distinct patterns of atrophy that correlate with disease burden. This demonstrates insights into the underlying disease biology and the potential value of categorizing patients in clinical trials.
Fig 1: SuStaIn identied two brain atrophy subtypes
Fig2: Phenoconversion correlates with stage
Table 1: normal scans had lower clinical burden
To cite this abstract in AMA style:
S. Joza, A. Delva, C. Tremblay, A. Vo, J-F. Gagnon, R. Postuma, P. Dusek, M. Hu, J. Klein, J-P. Taylor, J. O'Brien, M. Firbank, A. Thomas, P. Donaghy, S. Lehericy, I. Arnulf, M. Vidailhet, J-C. Corvol, M. Sommerauer, S. Röttgen, S. Lewis, E. Matar, K. Ehgoetz Martens, L. Churchill, R. Camicioli, H. Chertkow, P. Borghammer, K. Knudsen, A. Hansen, D. Arnaldi, B. Orso, P. Matiioli, A. Dagher, S. Rahayel. Identification of Brain Atrophy Subtypes in Prodromal and Overt Synucleinopathies: A Multicenter Study [abstract]. Mov Disord. 2024; 39 (suppl 1). https://www.mdsabstracts.org/abstract/identification-of-brain-atrophy-subtypes-in-prodromal-and-overt-synucleinopathies-a-multicenter-study/. Accessed July 5, 2025.« Back to 2024 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/identification-of-brain-atrophy-subtypes-in-prodromal-and-overt-synucleinopathies-a-multicenter-study/